Carotenoids are a large and diverse group of compounds that have been shown to have a wide range of potential health benefits. While some carotenoids have been extensively studied, numerous others have not received as much attention. In numerous studies, using EPR (electron paramagnetic resonance) techniques in correlation with DFT (density functional theory) calculations, researchers have characterized about 20 conventional carotenoids - their electron transfer from the carotenoid molecule to form the radical cation, and further proton loss from the radical cation to form neutral radicals (radicals with no charge). Several conventional carotenoids are briefly discussed here.
- xanthophylls
- electron transfer
- radical cation
- carotenoid neutral radicals
1. Introduction
2. Studies of Electron and Proton Loss of Conventional Carotenoids by DFT and EPR
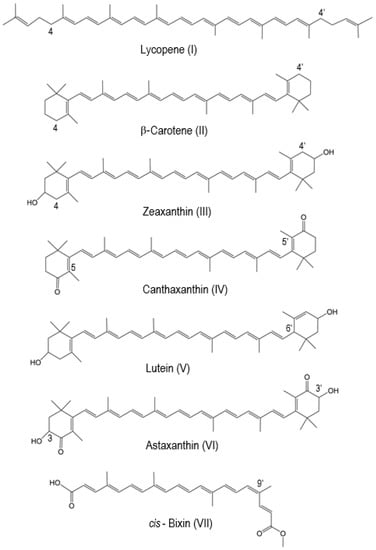
| Carotenoid | The Most Favorable Proton Loss from the Radical Cation | Reference |
|---|---|---|
| Lycopene | C4(4′) | [14] |
| β-carotene | C4(4′) | [15] |
| Zeaxanthin | C4(4′) | [15][16] |
| Canthaxanthin | C5(5′) | [15] |
| Lutein | C6′ | [15] |
| Astaxanthin | C3(C3′) | [17] |
| cis-Bixin | C9′ | [20] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms24129885
References
- Yabuzaki, J. Carotenoids database: Structures, chemical fingerprints and distribution among organisms. Database J. Biol. Databases Curation 2017, 2017, bax004.
- Wang, P.Y.; Fang, J.C.; Gao, Z.H.; Zhang, C.; Xie, S.Y. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. J. Diabetes Investig. 2016, 7, 56–69.
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056.
- Saini, R.K.; Rengasamy, K.R.; Mahomoodally, F.M.; Keum, Y.-S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020, 155, 104730.
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26.
- Castenmiller, J.J.M.; West, C.E. Bioavailability and bioconversion of carotenoids. Annu. Rev. Nutr. 1998, 18, 19–38.
- dela Seña, C.; Riedl, K.M.; Narayanasamy, S.; Curley, R.W., Jr.; Schwartz, S.J.; Harrison, E.H. The human enzyme that converts dietary provitamin A carotenoids to vitamin A is a dioxygenase. J. Biol. Chem. 2014, 289, 13661–13666.
- Collini, E. Carotenoids in photosynthesis: The revenge of the “accessory” pigments. Chem 2019, 5, 494–495.
- Hashimoto, H.; Uragami, C.; Cogdell, R.J. Carotenoids and photosynthesis. In Carotenoids in Nature; Stange, C., Ed.; Subcellular Biochemistry; Springer: Cham, Switzerland, 2016; Volume 79.
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42.
- Konovalova, T.A.; Krzystek, J.; Bratt, P.J.; van Tol, J.; Brunel, L.-C.; Kispert, L.D. 95–670 GHz EPR studies of canthaxanthin radical cation stabilized on a silica−Alumina surface. J. Phys. Chem. B 1999, 103, 5782–5786.
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Carotenoids: Importance in daily life—Insight gained from EPR and ENDOR. Appl. Magn. Reson. 2021, 52, 1093–1112.
- Focsan, A.L.; Kispert, L.D. Radicals formed from proton loss of carotenoid radical cations: A special form of carotenoid neutral radical occurring in photoprotection. J. Photochem. Photobiol. B Biol. 2017, 166, 148–157.
- Focsan, A.L.; Bowman, M.K.; Molnár, P.; Deli, J.; Kispert, L.D. Carotenoid radical formation: Dependence on conjugation length. J. Phys. Chem. B 2011, 115, 9495–9506.
- Lawrence, J.; Focsan, A.L.; Konovalova, T.A.; Molnar, P.; Deli, J.; Bowman, M.K.; Kispert, L.D. Pulsed electron nuclear double resonance studies of carotenoid oxidation in Cu(II)-substituted MCM-41 molecular sieves. J. Phys. Chem. B 2008, 112, 5449–5457.
- Focsan, A.L.; Bowman, M.K.; Konovalova, T.A.; Molnár, P.; Deli, J.; Dixon, D.A.; Kispert, L.D. Pulsed EPR and DFT characterization of radicals produced by photo-oxidation of zeaxanthin and violaxanthin on silica−alumina. J. Phys. Chem. B 2008, 112, 1806–1819.
- Focsan, A.L.; Bowman, M.K.; Shamshina, J.; Krzyaniak, M.D.; Magyar, A.; Polyakov, N.E.; Kispert, L.D. EPR study of the astaxanthin n-octanoic acid monoester and diester radicals on silica–alumina. J. Phys. Chem. B 2012, 116, 13200–13210.
- Rivera-Madrid, R.; Aguilar-Espinosa, M.; Cardenas-Conejo, Y.; Garza-Caligaris, L.E. Carotenoid derivates in achiote (Bixa orellana) seeds: Synthesis and health promoting properties. Front. Plant Sci. 2016, 7, 1406.
- Hernandez-Marin, E.; Galano, A.; Martínez, A. Cis carotenoids: Colorful molecules and free radical quenchers. J. Phys. Chem. B 2013, 117, 4050–4061.
- Tay-Agbozo, S.S.; Krzyaniak, M.D.; Bowman, M.K.; Street, S.; Kispert, L.D. DFT and ENDOR study of bixin radical cations and neutral radicals on silica–alumina. J. Phys. Chem. B 2015, 119, 7170–7179.
- Focsan, A.L.; Molnár, P.; Deli, J.; Kispert, L. Structure and properties of 9’-cis neoxanthin carotenoid radicals by electron paramagnetic resonance measurements and density functional theory calculations: Present in LHC II? J. Phys. Chem. 2009, 113, 6087–6096.
