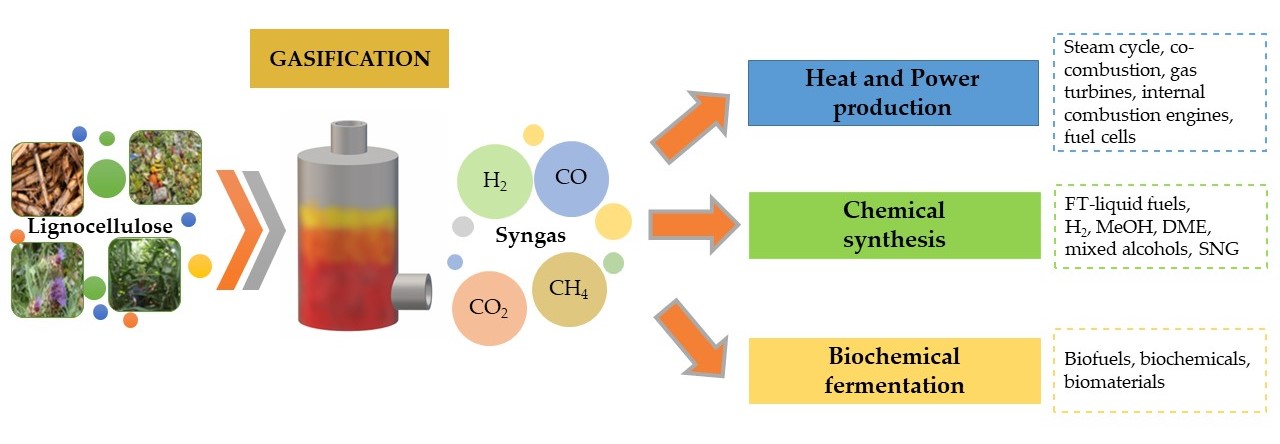
Lignocellulosic gasification is a valid thermochemical approach for the conversion of organic solid matter into a gaseous mixture that is constituted of hydrogen, carbon monoxide, carbon dioxide and methane, named synthetic gas or syngas. Although about 55% of syngas is still produced from coal, biomass utilization, especially lignocellulose, is constantly growing. Indeed, gasification could be potentially applied to all different kinds of lignocellulosic biomass, unlike other conversion technologies. Moreover, in the last few decades, a wide range of applications of syngas have been intensively studied. Syngas can be directly used as a combustible substance in power plants for heat and power production (steam cycle, co-combustion, combustion in gas turbines or internal combustion engines, high temperature fuel cells), which represents the most common use of biomass-derived syngas. However, syngas also represents a platform that can be employed in a broad range of chemical and microbial processes, leading to gaseous and liquid fuels, as well as to chemicals. Chemical process research has mainly focused on transportation fuel production from syngas, such as Fischer–Tropsch liquid fuels, hydrogen, methanol, dimethyl ether (DME), mixed alcohols, and synthetic natural gas (SNG). Instead, the biochemical conversion route consists of syngas fermentation in which obligate anaerobic microorganisms convert syngas into organic acids, alcohols, and other chemicals. The most commonly used microorganisms are acetogens, which use the Wood–Ljungdahl metabolic pathway. Syngas fermentation is defined as an indirect fermentation process because biomass is not fed directly into the fermenter, but it is previously converted into syngas through gasification.
- biomass gasification
- syngas
- thermochemical process
1. Introduction
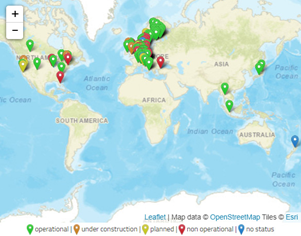
The gasification of carbonaceous feedstocks to syngas takes place inside a reactor, defined as a gasifier, at high temperatures (800–1500 °C). The feedstock is subjected to partial oxidation due to a lower concentration of oxygen than the stoichiometric requirement. Oxygen is supplied by a gasifying agent or carrier, such as air, pure oxygen, water steam, or their mixture. Although carbon dioxide can also be used as a gasifying agent, its use is less frequent. Moreover, the use of supercritical water is an innovative technology, without the need for pretreatment, which achieves a high H2 yield and reduces tar and char production[1][2][3]. Compared with conventional methods, gasification is a more efficient process than combustion, which is the most common thermochemical route [4], and it can convert the entire carbon content in the biomass feedstock into gaseous compounds, unlike the biological or chemical hydrolysis that is adopted in biochemical processes [5]. According to the IEA Bioenergy Task 33, there are 114 working biomass gasification projects worldwide, 15 plants idle or on hold, and 13 are under construction or in planning (Figure 1) [6].
Figure 1. Geographical distribution of biomass gasification projects [6].
2. Syngas Characteristics By Biomass Type
At the end of the entire process, two main product mixtures are present: a solid mixture and a gaseous mixture. The solid mixture contains the unreacted organic fraction and inert materials, such as tars and ashes. Tars are classified into primary, secondary, and tertiary tars. Primary tars consist of both oxygenated compounds (alcohols, carboxylic acids, ketones, aldehydes, etc.) and substituted phenols (cresol, xylenol, etc.). Secondary tars are alkylated aromatics, such as toluene, ethylbenzene, xylenes, styrene, and hetero-aromatics, such as pyridine, furan, dioxin, and thiophene. Finally, tertiary tars consist of aromatics and polycyclic aromatic hydrocarbons (PAH), such as benzene, naphthalene, phenanthrene, pyrene, and benzopyrene. While primary tars are produced directly from the pyrolysis of cellulose, hemicellulose, and lignin, secondary and tertiary tars are the result of several complex reactions that have not been fully clarified yet[7]. On the other hand, the gaseous mixture contains syngas (H2, CO, CO2, and CH4) and a small amount of impurities, such as light hydrocarbons (ethane, ethylene, acetylene), hydrogen sulfide (H2S), sulfur dioxide (SO2), hydrogen chloride (HCl), nitrogen oxides (NOx), nitrogen (N2), and ammonia (NH3)[8]. The syngas’s final composition and characteristics are related to the type of biomass, gasifying agent, gasifier type, and reactor’s operational conditions, such as temperature, pressure, equivalence ratio (ER), residence time, and catalyst used [2][9][10][11][12][13]. For these reasons, in the last few decades, gasification has been intensively studied to investigate the effects of these factors, and thus, to identify the optimal conditions for the process. Regarding feedstock type, wood is the most commonly used feedstock in the gasification process. A representative component profile for syngas produced from several woody biomass types is shown in Table 1. In addition to woody biomass, other kinds of biomass have also been studied as gasification feedstocks. Agro-industrial residues and perennial herbaceous crops (Table 2) represent promising feedstocks that can be used in a thermochemical conversion process to obtain both energy and chemicals. The use of agricultural and industrial wastes, as well as herbaceous crops, instead of woody feedstocks, extends the seasonal availability of biomass. Syngas’s composition is highly dependent on the used feedstocks, as well as the gasification technology applied (Tables 1 and 2). It is worth pointing out that nitrogen (N2) can represent a main syngas component when air is used as a gasifying agent, in addition to H2, CO, CO2, and CH4. Therefore, air gasification results in N2-diluted syngas with low H2 and CO concentrations. Instead, when gasification is carried out with steam or oxygen, the syngas shows higher H2 and CO concentrations. One of the major challenges in biomass gasification is producing syngas with a low or absent impurities content.
Table 1. Syngas compositions that are obtained from the gasification of several woody biomass and their process characteristics.
|
Feedstock |
Syngas Composition (% v/v) |
Gasifier Type |
Gasification Conditions |
Reference |
|||
|
H2 |
CO |
CO2 |
CH4 |
||||
|
Mesquite wood |
1.6–3.0 |
13.0–21.0 |
11.0–25.0 |
1.0–1.5 |
Fixed bed gasifier |
GA: air; T: 782 °C; ER: 2.70 |
[15] |
|
Juniper wood |
2.5–3.5 |
21.0–25.0 |
9.0–12.0 |
1.5–1.8 |
Fixed bed gasifier |
GA: air; T: 713 °C; ER: 2.70 |
[15] |
|
Pine wood |
30.5 |
52.8 |
14.7 |
2.0 |
Downdraft fixed bed gasifier |
GA: steam; T: 900 °C; ER: N.A. |
[16] |
|
Oak wood |
18.0 |
21.0 |
12.0 |
2.0 |
Downdraft fixed bed gasifier |
GA: air; T: N.A.; ER: N.A. |
[17] |
|
Poplar wood |
45.5 |
23.1 |
20.8 |
8.6 |
Rotary kiln reactor |
GA: steam; T: 1500 °C; ER: N.A. |
[18] |
|
Eucalyptus wood |
10.7 |
20.2 |
9.1 |
8.6 |
Downdraft fixed bed gasifier |
GA: air; T: 865 °C; ER: 0.31 |
[19] |
|
Coffee wood |
12.4 |
14.0 |
10.4 |
6.5 |
Downdraft fixed bed gasifier |
GA: air; T: 813 °C; ER: 0.32 |
[19] |
|
Rubber wood |
6.0–8.0 |
10.0–14.0 |
16.0–18.0 |
N.A. |
Bubbling fluidized bed gasifier |
GA: air; T: 750–900 °C; ER: 0.38 |
[20] |
|
Oil palm wood |
60.0–70.0 |
10.0–30.0 |
20.0–50.0 |
5.0–10.0 |
N.A. |
GA: steam; T: 800 °C; ER: N.A. |
[21] |
|
Spruce wood |
10.7 |
25.9 |
9.7 |
3.8 |
Fixed bed reactor |
GA: air; T: 800 °C; ER: N.A. |
[22] |
|
Wood residue |
42.5 |
23.0 |
18.1 |
11.5 |
Fluidized bed gasifier |
GA: air; T: 823 °C; ER: 0.17 |
[23] |
|
Vermont wood a |
28.6 |
23.5 |
24.0 |
15.5 |
Fluidized bed gasifier |
GA: steam; T: 600–710 °C; ER: N.A. |
[24] |
|
Wood residue b |
26.2–28.0 |
50.0–60.3 |
12.7–23.3 |
0.9–1.8 |
Entrained flow gasifier |
GA: oxygen; T: 1200–1500 °C; ER: 0.44 |
[25] |
|
SRF wood c |
15.7–16.5 |
15.9–17.2 |
14.3–15.1 |
2.6–2.7 |
Downdraft fixed bed reactor |
GA: air; T: 650–800 °C; ER: 0.25–0.26 |
[26] |
|
Wood waste d |
9.4–14.8 |
15.1–19.4 |
11.0–15.8 |
3.2–4.3 |
Bubbling fluidized bed gasifier |
GA: air/air and steam mixture; T: 850 °C; ER: 0.20–0.29 |
[27] |
GA: gasifying agent, T: temperature, ER: equivalence ratio, N.A.: data not available. a Vermont wood is a mixture of 25% red oak, 15% white pine, 15% maple, 15% ash, and 10% poplar, with the balance being cherry, birch, and cedar. b Wood residue is a mixture of 45% hardwood (birch) and 55% softwood (pine). c Solid recovered fuels (SRF) wood is composed of waste furniture and waste pallets from a waste collection site. d Wood waste that cannot be utilized to produce fuel for domestic heating because they come from potentially contaminated waste; it is made of sawdust from the wood packaging industry or it is obtained as a recycled product from furniture and from door and window frames.
Table 2. Syngas compositions obtained from the gasification of several agro-industrial residues and herbaceous crops and their process characteristics.
|
Feedstock |
Syngas Composition (% v/v) |
Gasifier Type |
Gasification Conditions |
Ref. |
|||
|
H2 |
CO |
CO2 |
CH4 |
||||
|
Corn straw |
48.5 |
33.9 |
12.2 |
5.3 |
N.A. |
GA: N.A.; T: 750–900 °C; ER: N.A. |
[28] |
|
Wheat straw |
25.4 |
27.5 |
22.0 |
16.3 |
Fluidized bed gasifier |
GA: steam; T: 600–710 °C; ER: N.A. |
[24] |
|
Rice husk |
5.0–8.0 |
16.0–21.0 |
15.0–16.0 |
46.0 |
Fluidized bed gasifier |
GA: air; T: 700–800 °C; ER: 0.18–0.27 |
[29] |
|
Coffee husk |
6.6 |
13.8 |
12.1 |
14.8 |
Downdraft fixed bed gasifier |
GA: air; T: 669 °C; ER: 0.12 |
[19] |
|
Coconut coir |
7.0–21.4 |
18.6–20.3 |
19.1–21.3 |
6.1–9.0 |
Entrained flow reactor |
GA: air; T: 726–941 °C; ER: 0.21–0.30 |
[30] |
|
Groundnut shells |
13.8 |
13.0 |
13.5 |
5.7 |
Bubbling fluidized bed gasifier |
GA: air; T: 714.4 °C; ER: 0.31 |
[31] |
|
Almond shells |
34.2–39.6 |
17.8–23.2 |
10.7–16.8 |
N.A. |
Bubbling fluidized bed reactor |
GA: N.A.; T: 820 °C; ER: N.A. |
[32] |
|
Hazelnut shells |
11.1–14.7 |
8.6–20.7 |
9.5–16.3 |
1.4–2.5 |
Downdraft fixed bed gasifier |
GA: air; T: 1000–1050 °C; ER: N.A. |
[33] |
|
Hay |
8.8 |
19.7 |
14.4 |
3.0 |
Fixed bed reactor |
GA: air; T: 800 °C; ER: N.A. |
[22] |
|
Corn stover |
26.9 |
24.7 |
23.7 |
15.3 |
Fluidized bed gasifier |
GA: steam; T: 600–710 °C; ER: N.A. |
[24] |
|
Olive kernels |
5.4–9.3 |
6.9–8.6 |
19.0–21.7 |
1.8–3.0 |
Circulating fluidized bed gasifier |
GA: air; T: 800 °C; ER: 0.4–0.7 |
[34] |
|
Vine pruning |
17.1–18.4 |
21.3–21.7 |
11.3–13.0 |
2.1–2.6 |
Downdraft fixed bed reactor |
GA: air; T: N.A.; ER: 0.26 |
[35] |
|
Corncobs |
17.3 |
22.6 |
12.0 |
1.98 |
Downdraft fixed bed reactor |
GA: air; T: N.A.; ER: 0.28 |
[36] |
|
Citrus peels |
60.0–65.0 |
15.0–25.0 |
15.0–23.0 |
<5.0 |
Fixed bed gasifier |
GA: steam; T: 750 °C; ER: N.A. |
[37] |
|
Posidonia oceanica |
11.8–24.9 |
4.1–12.7 |
14.1–20.0 |
2.0–3.0 |
Fluidized bed gasifier |
GA: air; T: 750 °C; ER: 0.3 |
[38] |
|
Empty fruit brunch |
12.9–13.5 |
17.0–17.4 |
13.7–14.5 |
1.5–1.9 |
Downdraft fixed bed gasifier |
GA: air; T: 650–825 °C; ER: N.A. |
[39] |
|
Sugarcane bagasse |
7.4–8.0 |
8.0–12.9 |
15.9–18.7 |
1.4–2.5 |
Cyclone gasifier |
GA: air; T: 600–950 °C; ER: 0.18–0.25 |
[40] |
|
Sewage sludge |
5.1–8.1 |
19.5–31.6 |
13.3–16.5 |
0.9–1.5 |
Fixed-bed gasifier |
GA: air; T: 650–1100 °C; ER: 0.12–0.27 |
[41] |
|
Miscanthus X giganteus |
8.6 |
16.4 |
14.0 |
4.4 |
Bubbling fluidized bed reactor |
GA: air; T: 800 °C; ER: 0.21 |
[42] |
|
Switchgrass (Panicum vigatum) |
23.5 |
33.2 |
19.4 |
17.0 |
Fluidized bed gasifier |
GA: steam; T: 600–710 °C; ER: N.A. |
[24] |
|
Thistle (Cynara cardunculus L.) |
36.6 |
8.5 |
50.4 |
4.5 |
Circulating fluidized bed gasifier |
GA: steam and oxygen; T: 750 °C; ER: 0.3 |
[43] |
|
Wheatgrass (Elytrigia elongata) |
10.8 |
12.3 |
16.5 |
5.3 |
Bubbling fluidized bed gasifier |
GA: oxygen-enriched air; T: 800 °C; ER: N.A. |
[44] |
GA: gasifying agent. T: temperature. ER: equivalence ratio. N.A.: not available data.
3. Syngas Final Uses
Syngas, depending on its composition and final quality, is used as (Figure 2):
-
combustible substance to produce heat and power
-
platform based on chemical synthesis of Fischer-Tropsch (FT) liquid fuels, hydrogen (H2), methanol (MeOH), dimethyl ether (DME), mixed alcohols and synthetic natural gas (SNG)
-
platform based on biochemical fermentation to produce (bio)chemicals, (bio)fuels and (bio)materials.
Future investigation studies on biomass gasification will be strategic to achieve an ideal syngas composition, thereby making the syngas final use as efficient as possible[14].
This entry is adapted from the peer-reviewed paper 10.3390/pr8121567
References
- Neubauer, Y.; Liu, H.; Biomass gasification. Biomass Combust. Sci. Technol. Eng. 2013, 8, 106–129, 10.1533/9780857097439.2.106.
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S.; An overview of advances in biomass gasification.. Energy Environ. Sci. 2016, 9, 2939–2977, .
- Sansaniwal, S.K.; Pal, K.; Rosen, M.A.; Tyagi, S.K.; Recent advances in the development of biomass gasification technology: A comprehensive review. . Renew. Sustain. Energy Rev. 2017, 72, 363–384, .
- Dai, J.; Saayman, J.; Grace, J.R.; Ellis, N.; Gasification of Woody Biomass. Annu. Rev. Chem. . Biomol. Eng. 2015, 6, 77–99, .
- Kennes, D.; Abubackar, H.N.; Diaz, M.; Veiga, M.C.; Kennes, C.; Bioethanol production from biomass: Carbohydrate vs syngas fermentation. . J. Chem. Technol. Biotechnol. 2016, 91, 304–317, .
- IEA BioEnergy Agreement Task 33: Thermal Gasification of Biomass . IEA BioEnergy Agreement Task 33. Retrieved 2020-12-3
- Molino, A.; Chianese, S.; Musmarra, D.; Biomass gasification technology: The state of the art overview.. J. Energy Chem. 2016, 25, 10–25, .
- Xu, D.; Tree, D.R.; Lewis, R.S.; The effects of syngas impurities on syngas fermentation to liquid fuels.. Biomass Bioenergy 2011, 35, 2690–2696, .
- Ciferno, J.P.; Marano, J.J. Benchmarking biomass gasification technologies for fuels, chemicals andhydrogen production; US DOE National Energy Technology Laboratory: Pittsburgh, PA, USA, June 2002
- Kruse, A.; Supercritical water gasification.. Biofuels Bioprod. Biorefining 2008, 2, 415–437, .
- Yang, H.; Chen, H. Biomass Gasification for Synthetic Liquid Fuel Production; All rights reserved; WoodheadPublishing Limited: Sawston, UK, 2015; ISBN 9780857098085
- Cheng, Y.; Thow, Z.; Wang, C.H. Biomass gasification with CO2 in a fluidized bed. Powder Technol. 2016,296, 87–101.
- Ren, J.; Cao, J.P.; Zhao, X.Y.; Yang, F.L.; Wei, X.Y.; Recent advances in syngas production from biomass catalytic gasification: A critical review on reactors, catalysts, catalytic mechanisms and mathematical models. . Renew. Sustain. Energy Rev. 2019, 116, 109426, .
- Ramachandriya, K.D.; Kundiyana, D.K.; Sharma, A.M.; Kumar, A.; Atiyeh, H.K.; Huhnke, R.L.; Wilkins, M.R.; Critical factors affecting the integration of biomass gasification and syngas fermentation technology.. AIMS Bioeng. 2016, 3, 188–210, .
- Chen, W.; Annamalai, K.; Ansley, R.J.; Mirik, M.; Updraft fixed bed gasification of mesquite and juniper wood samples. . Energy 2012, 41, 454–461, .
- Huang, F.; Jin, S.; Investigation of biomass (pine wood) gasification: Experiments and Aspen Plus simulation. . Energy Sci. Eng. 2019, 7, 1178–1187, .
- Yan, Q.; Yu, F.; Liu, J.; Street, J.; Gao, J.; Cai, Z.; Zhang, J. Catalytic conversion wood syngas to syntheticaviation turbine fuels over a multifunctional catalyst. Bioresour. Technol. 2013, 127, 281–290.
- Galvagno, S.; Casciaro, G.; Casu, S.; Martino, M.; Mingazzini, C.; Russo, A.; Portofino, S. Steam gasificationof tyre waste, poplar, and refuse-derived fuel: A comparative analysis. Waste Manag. 2009, 29, 678–689.
- de Oliveira, J.L.; da Silva, J.N.; Martins, M.A.; Pereira, E.G. Gasification of waste from coffee and eucalyptusproduction as an alternative source of bioenergy in Brazil. Sustain. Energy Technol. Assess. 2018, 27, 159–166.
- Kaewluan, S.; Pipatmanomai, S. Potential of synthesis gas production from rubber wood chip gasificationin a bubbling fluidised bed gasifier. In Energy Conversion and Management; Elsevier Ltd.: Amsterdam, TheNetherlands, 2011; Volume 52, pp. 75–84.
- Nipattummakul, N.; Ahmed, I.I.; Kerdsuwan, S.; Gupta, A.K.; Steam gasification of oil palm trunk waste for clean syngas production.. Appl. Energy 2012, 92 , 778–782, .
- Mikeska, M.; Najser, J.; Peer, V.; Frantík, J.; Kielar, J.; Quality assessment of gas produced from different types of biomass pellets in gasification process. . Energy Explor. Exploit. 2020, 38, 406–416, .
- Peng, W.X.; Wang, L.S.; Mirzaee, M.; Ahmadi, H.; Esfahani, M.J.; Fremaux, S. ,; Hydrogen and syngas production by catalytic biomass gasification.. Energy Convers. Manag. 2017, 135, 270–273, .
- Carpenter, D.L.; Bain, R.L.; Davis, R.E.; Dutta, A.; Feik, C.J.; Gaston, K.R.; Jablonski, W.; Phillips, S.D.; Nimlos, M.R.; Pilot-scale gasification of corn stover, switchgrass, wheat straw, and wood: 1. Parametric study and comparison with literature. . Ind. Eng. Chem. Res. 2010, 49, 1859–1871, .
- Weiland, F.; Nordwaeger, M.; Olofsson, I.; Wiinikka, H.; Nordin, A.; Entrained flow gasification of torrefied wood residues.. Fuel Process. Technol. 2014, 125, 51–58, .
- Vonk, G.; Piriou, B.; Dos Santos, P.F.; Wolbert, D.; Vaïtilingom, G.; Comparative analysis of wood and solid recovered fuels gasification in a downdraft fixed bed reactor. . Waste Manag. 2019, 85, 106–120, .
- Arena, U.; Zaccariello, L.; Mastellone, M.L.; Gasification of Natural and Waste Biomass in a Pilot Scale Fluidized Bed Reactor.. Combust. Sci. Technol. 2010, 182 2010, 182, 625–639, .
- Hu, P.; Chakraborty, S.; Kumar, A.; Woolston, B.; Liu, H.; Emerson, D.; Stephanopoulos, G.; Integrated bioprocess for conversion of gaseous substrates to liquids. . Proc. Natl. Acad. Sci. USA 2016, 113, 3773–3778, .
- Wu, C.Z.; Yin, X.L.; Ma, L.L.; Zhou, Z.Q.; Chen, H.P.; Operational characteristics of a 1.2-MW biomass gasification and power generation plant.. Biotechnol. Adv. 2009, 27, 588–592, .
- Senapati, P.K.; Behera, S.; Experimental investigation on an entrained flow type biomass gasification system using coconut coir dust as powdery biomass feedstock. . Bioresour. Technol. 2012, 117, 99–106, .
- Singh, D.; Yadav, S.; Rajesh, V.M.; Mohanty, P.; Groundnut shell gasification performance in a fluidized bed gasifier with bubbling air as gasification medium. . Environ. Technol. 2019, 40, 3140–3152, .
- Rapagnà, S.; Steam-gasification of biomass in a fluidised-bed of olivine particles. . Biomass Bioenergy 2000, 19, 187–197, .
- Dogru, M.; Howarth, C.R.; Akay, G.; Keskinler, B.; Malik, A.A.; Gasification of hazelnut shells in a downdraft gasifier. . Energy 2002, 27, 415–427, .
- García-Ibañez, P.; Cabanillas, A.; Sánchez, J.M.; Gasification of leached orujillo (olive oil waste) in a pilot plant circulating fluidised bed reactor. Preliminary results. . Biomass Bioenergy 2004, 27, 183–194, .
- Biagini, E.; Barontini, F.; Tognotti, L.; Gasification of agricultural residues in a demonstrative plant: Corn cobs. . Bioresour. Technol. 2015, 173, 110–116, .
- Biagini, E.; Barontini, F.; Tognotti, L.; Gasification of Agricultural Residues in a Demonstrative Plant. . Bioresour. Technol. 2014, 37, 110–116, .
- Chiodo, V.; Urbani, F.; Zafarana, G.; Prestipino, M.; Galvagno, A.; Maisano, S.; Syngas production by catalytic steam gasification of citrus residues. . Int. J. Hydrogen Energy 2017, 42, 28048–28055, .
- Maisano, S.; Urbani, F.; Cipitì, F.; Freni, F.; Chiodo, V.; Syngas production by BFB gasification: Experimental comparison of different biomasses. . Int. J. Hydrogen Energy 2019, 44, 4414–4422, .
- Erlich, C.; Fransson, T.H.; Downdraft gasification of pellets made of wood, palm-oil residues respective bagasse: Experimental study. . Appl. Energy 2011, 88, 899–908, .
- Gabra, M.; Pettersson, E.; Backman, R.; Kjellström, B.; Evaluation of cyclone gasifier performance for gasification of sugar cane residue - Part 1: Gasification of bagasse. . Biomass Bioenergy 2001, 21, 351–369, .
- Werle, S.; Impact of feedstock properties and operating conditions on sewage sludge gasification in a fixed bed gasifier. . Waste Manag. Res. 2014, 32, 954–960, .
- Xue, G.; Kwapinska, M.; Horvat, A.; Kwapinski, W.; Rabou, L.P.L.M.; Dooley, S.; Czajka, K.M.; Leahy, J.J.; Gasification of torrefied Miscanthus×giganteus in an air-blown bubbling fluidized bed gasifier. . Bioresour. Technol. 2014, 159, 397–403, .
- Christodoulou, C.; Tsekos, C.; Tsalidis, G.; Fantini, M.; Panopoulos, K.D.; De Jong, W.; Kakaras, E.; Attempts on cardoon gasification in two different circulating fluidized beds. . Case Stud. Therm. Eng. 2014, 4, 42–52, .
- Torreiro, Y.; Ortiz, I.; Molina, G.; Maroño, M.; Pérez, V.; Murillo, J.M.; Ramos, R.; Fernández, M.; García, S.; Sánchez, J.M.; et al. Thermochemical assessment of Nicotiana glauca, Panicum virgatum and Elytrigia elongata as fuels for energy recovery through gasification. . Fuel 2018, 225, 71–79, .
- Christodoulou, C.; Tsekos, C.; Tsalidis, G.; Fantini, M.; Panopoulos, K.D.; De Jong, W.; Kakaras, E.; Attempts on cardoon gasification in two different circulating fluidized beds. . Case Stud. Therm. Eng. 2014, 4, 42–52, .
- Torreiro, Y.; Ortiz, I.; Molina, G.; Maroño, M.; Pérez, V.; Murillo, J.M.; Ramos, R.; Fernández, M.; García, S.; Sánchez, J.M.; et al. Thermochemical assessment of Nicotiana glauca, Panicum virgatum and Elytrigia elongata as fuels for energy recovery through gasification. . Fuel 2018, 225, 71–79, .