Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nanoscience & Nanotechnology
Ferroptosis is a new form of iron-dependent programmed cell death discovered, which is caused by the accumulation of lipid peroxidation (LPO) and reactive oxygen species (ROS). Studies have shown that cellular ferroptosis is closely related to tumor progression, and the induction of ferroptosis is a new means to inhibit tumor growth.
- Fe3O4 nanoparticles
- ferroptosis
1. Introduction
Global Statistics of 2020 [1] showed that there were an estimated 19.3 million new cancer cases and nearly 10 million cancer deaths, of which female breast cancer surpassed lung cancer as the most commonly diagnosed cancer with an estimated 2.3 million new cases (11.7%), followed closely by lung cancer (11.4%), colorectal cancer (10.0%), prostate cancer (7.3%), and stomach cancer (5.6%). Lung cancer remained the leading cause of cancer deaths with an estimated 1.8 million deaths (18%), followed by colorectal cancer (9.4%), liver cancer (8.3%), stomach cancer (7.7%), and female breast cancer (6.9%). The proliferation and apoptosis of corresponding tumor cells are closely related to tumor formation and progression. Thus, in the past few decades in oncology, scientific researchers have been devoted to the development of various apoptosis-related drugs to reverse the fate of non-senescent and over-proliferating tumor cells [2,3,4].
However, the therapeutic outcome is far from satisfactory due to the inherent or acquired apoptosis resistance of tumor cells [5]. Lung cancer, with the highest age-standardized incidence rate (ASIR) and age-standardized mortality rate (ASMR) worldwide, and with 2.1 million new cases and 1.8 million deaths every year, has a very high mortality rate [6,7,8,9]. Small cell lung cancer (SCLC) is one of the most malignant types of lung cancer. It has a short multiplication time, patients are prone to rapid drug resistance during treatment, and the disease deteriorates rapidly after recurrence. Currently, there is no effective second-line single-agent chemotherapy regimen other than topotecan [10]. In addition, the incidence and mortality of hepatocellular carcinoma (HCC) has rapidly increased worldwide [11]. HCC is an aggressive and highly malignant liver tumor with poor survival rates, and most patients are clinically diagnosed at an advanced stage [12]. The pathogenesis of the tumor has been of great interest to researchers; however, due to its diversity and complexity, it still has a long way to go despite the large amount of human and material resources invested every year [13,14,15,16,17]. In recent years, a new form of cell death different from the apoptotic mechanism, namely ferroptosis, has attracted attention.
Ferroptosis, first proposed in 2012 by Scott J. Dixon et al., is a unique form of iron-dependent non-apoptotic cell death triggered by the oncogenic RAS-selective lethal small molecule Erastin [18]. Early studies found that cysteine is required for the growth and death inhibition of cells, and the absence of cystine in the culture medium resulted in the death of human fibroblasts due to glutathione (GSH) depletion. Extracellular and intracellular cysteine are required to maintain GSH biosynthesis and inhibit mammalian cell death, which could be prevented and treated with the lipophilic antioxidant (α-tocopherol) and iron chelators (deferoxamine) [19]. In the following years, with the further study of the mechanism of ferroptosis, it has gradually become a promising cancer treatment.
Compared with other metal nanomaterials [20,21,22,23], Fe3O4-NPs are one of the few FDA-approved nanomaterials for clinical use, which has simple preparation [24,25,26], safe and stable chemical properties, and good biocompatibility [27,28,29]. Moreover, it has been widely used in pathogen detection [30,31,32,33,34], tumor diagnosis and treatment [35,36,37], gene mutation analysis [38,39,40,41,42], targeted drug delivery [43,44,45], and nuclear magnetic resonance imaging (MRI) [46,47,48]. In addition, recent studies have shown that Fe3O4-NPs, due to their rich Fe2+ and Fe3+ contents, can affect the iron metabolism of tumor cells, increase local ROS levels in tumor tissues, participate in or even induce the occurrence of ferroptosis in tumor cells, and then be able to inhibit or kill tumor cells.
2. Ferroptosis
Ferroptosis, a new form of cell death discovered in recent years, is an iron-dependent non-apoptotic form of cell death characterized by the accumulation of intracellular ROS [18]. This modality disrupts the redox homeostasis of cells and has a great potential to kill cancer cells [49,50]. Studies on the mechanisms of ferroptosis have focused on lipid peroxidation (LPO) and iron loading. Under normal circumstances, ROS is mainly produced by normal metabolism in mitochondria and is essential for cell signaling and tissue homeostasis [51]. Polyunsaturated fatty acid (PUFA) peroxidation induced by ROS is the main cause of ferroptosis. Furthermore, ferroptosis is regulated by a complex interaction between cellular redox homeostasis, lipid metabolism, and iron metabolism [52]. In terms of cell morphology, cells undergoing ferroptosis show some specific changes, such as the appearance of smaller mitochondria than normal, wrinkled mitochondrial membranes, reduced or absent mitochondrial cristae, and fragmented extracellular membrane [53]. The biochemical features of ferroptosis are cystine deficiency, GSH depletion, and glutathione peroxidase 4 (GPX4) inactivation [54]. However, the underlying mechanisms have not been fully elucidated.
The main cause of ferroptosis is the change of cell metabolism, which leads to the accumulation of LPO and ROS in the cells. According to existing research reports, the changes in cell metabolic pathways caused by ferroptosis mainly include the following aspects (Figure 1):
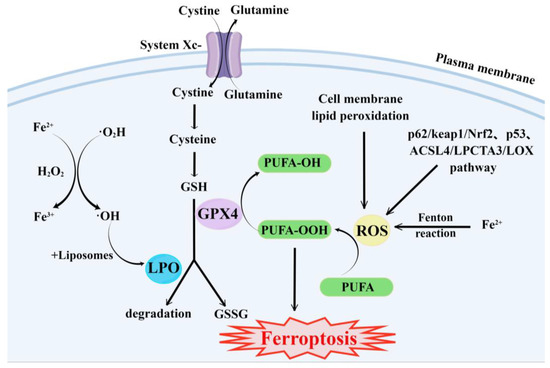
Figure 1. Major metabolic pathways of ferroptosis. By Figdraw.
- (1)
-
GSH synthesis pathway is inhibited and LPO accumulates, thus inducing ferroptosis in cells [55].
- (2)
-
Iron metabolism is altered. Iron is a redox-active metal involved in ROS formation and LPO diffusion, and a rising iron level could increase cellular susceptibility to iron-dependent cell death [56]. The accepted explanation today is that Fe2+ can transfer electrons to intracellular oxygen, and then react with intracellular lipids to form LPO, which further induces ferroptosis [57,58]. Iron metabolism genes and iron metabolism regulation genes play a key role in intracellular system iron homeostasis. For example, the gene of Iron Responsive Element Binding Protein 2 (IREB2) is a key player in the Erastin-induced ferroptosis of HT-1080 fibrosarcoma cells and Calu-1 lung cancer cells [59]. Thus, intracellular iron overload is critical for ferroptosis [60].
- (3)
-
ROS metabolic pathway effects. This pathway also plays an important role in ferroptosis. Cytosolic cystine/glutamate transport receptor (System Xc-) and voltage-dependent anion channels (VDACs) [18] in the outer mitochondrial membrane, GPX4 and ferroptosis suppressor protein 1 (FSP1) ferroptosis-related proteins [61,62], and p62/keap1/Nrf2 [63], p53-related pathway [64,65], and ACSL4/LPCTA3/LOX [66] ferroptosis-related pathways play their roles in regulating ferroptosis by affecting ROS metabolism pathways [67].
This entry is adapted from the peer-reviewed paper 10.3390/molecules28114562
This entry is offline, you can click here to edit this entry!
