1. Print-Light-Synthesis of Metal Nanoparticles, Nanostructures and Films
Combined inkjet printing and exposure to high intensity pulsed flashlight of thin liquid metal precursor films for the synthesis of metal nanoparticles was first introduced by Lesch [
3]. He fabricated platinum nanoparticles (NPs) and nanostructures as electrocatalysts on indium tin oxide (ITO)-coated glass slides. ITO was used as electrocatalyst support, one of the materials that is currently in discussion to replace carbon black, which in energy-related electrochemical devices can suffer from corrosion. Thanks to the high standard redox potential, Pt cations can be reduced under relatively mild conditions. Hydrogen hexachloroplatinate (H
2PtCl
6) was selected as a metal precursor due to its solubility in aqueous-based inks and the absence of alkali ions that would remain in the printed and light-processed layer in case potassium hexachloroplatinate (K
2PtCl
6) would be used. H
2PtCl
6 was dissolved in a mixture of water, isopropanol, and 1,2-propanediol in order to fulfill (i) the required physicochemical ink characteristics, such as surface tension and viscosity for printing, as well as good wetting of the substrate surface to create homogeneous and highly resolved patterns, and (ii) the presence of reducing agents, such as alcoholic compounds, to facilitate the reduction of Pt
IV to Pt
0. The photochemical and photothermal processes of Pt
IVCl
62− generated Pt
0 on the substrate notably by just one single light flash and, further, only gaseous side products, such as evaporated ink solvents, HCl, CO
2, and other volatile components.
It was further demonstrated by the author that the reduction of PtIV also took place using just pure water as solvent, thus without the presence of alcohols. It was further confirmed that the metal precursor film absorbed the light and generated the required heat and that it was not the substrate, which absorbed the light for heat generation. To demonstrate this, the author fabricated a pattern of Pt on quartz glass, which was transparent for the wavelengths emitted by the flashlamp. By using spectroscopic analyses (in particular XPS, XRD, and EDS), the complete precursor conversion was confirmed, in particular by the absence of chlorine signals in the printed Pt patterns. ITO-coated glass was selected as the substrate, not only because of its low absorption of the used wavelengths but also because it is conductive, enabling the use of the created Pt/ITO film as an electrode for electrochemical measurements.
Apart from creating nanoparticle-decorated electrodes, complete stand-alone thin-film metal electrodes are also of interest for electrochemical applications, as they do not require a conductive support and can be printed directly on an insulating substrate. In a very recent work, Maiorano, Gianvittorio et al. applied Print-Light-Synthesis to generate thin gold films as electrodes on a flexible substrate using an inkjet printer in combination with a mercury vapor short arc lamp [
26]. The advantage of using this lamp over the flashlamp is its smaller size and lower costs. A liquid light guide can be used to transport the light from the mercury lamp directly to the printhead, enabling simultaneous printing and photochemical reactions. Inkjet printing was used to generate the thin liquid reaction film with variable gold precursor loading. By using a UV absorbing substrate, in their case polyimide (PI), the substrate transformed light into heat, which was transferred in the above Au precursor ink. Therefore, Print-Light-Synthesis of Au on PI was based on a mixed photochemical and photo-induced thermal reduction process.
The Au electrodes were produced either as single nanoparticles on a previously printed conductive graphene layer or as a conductive gold film electrode directly on PI. Alternative substrates were glassy carbon and ITO-coated glass. The Au layers were well adhered and demonstrated clear signals for the electrochemical detection of glucose, 1,4-butanediol, and ascorbic acid.
In fact, obtaining conductive metal films using ink deposition based printing and the irradiation of metal precursors is challenging because often inhomogeneously distributed metal nanoparticle aggregates and/or very porous metal layers are generated that do not fulfill the requirements of many applications. In an earlier approach to preparing conductive metal films from metal precursor patterns involving light, Valeton et al. inkjet-printed an Ag precursor ink based on silver neodecanoate, which first had to be activated with UV light and then further chemically reduced by using hydroquinone as the reducing agent [
27]. Zope et al. inkjet-printed a solid silver-ligand complex, i.e., μ-oxolato-bis(ethylenediaminesilver(I)), on various substrates and compared thermal reduction on a hot plate and flash light-induced thermal reduction to obtain conductive Ag
0 patterns [
28].
Another research and development focus was the fabrication of Cu patterns. Even though Cu is an unusual bulk electrode material for electrochemistry, it can be interesting as films of individual nanoparticles for electrocatalysis. A flashlight was used to reduce printed Cu precursor patterns (of inorganic as well as organic origin) into conductive Cu
0 patterns in various ways, often to fabricate Cu traces for electronic circuits [
17,
29,
30,
31,
32,
33]. Song et al. demonstrated the importance of the ink composition in terms of solvents and stabilizers, such as ethyl cellulose and PVP, to obtain reasonable pattern resolution with homogeneous coverage using screen-printing [
30]. Copper in general oxidizes quickly in air to copper oxide, which has a reduced conductivity compared to Cu
0. Instead of thermal processing in an inert or reducing atmosphere, flashlight in the presence of reducing agents in air was used to obtain Cu
0 patterns free of Cu oxides, demonstrating another time the high potential of flashlight-based nanoparticle synthesis.
Patterning methods alternative to ink- or paste-based printing approaches (which create 2D precursor ink patterns with micrometer lateral pattern resolution) include the localized irradiation of a large liquid phase containing a dissolved metal precursor and either completely covering the target substrate or into which the target substrate is immersed. Compared to Print-Light-Synthesis based on inkjet printing, only the irradiation parameters are controllable and not the localized precursor loading and/or precursor film thickness. Localized irradiation of the precursor film is realized by translating a laser beam following the shape of the desired pattern or by using masks.
The aim of using this approach is generally to fabricate complete conductive metal films and also 3D structures for electronic applications. After light exposure, the non-converted metal precursor must be washed away, representing an additional process step compared to Print-Light-Synthesis involving inkjet printing. Based on the process, the substrate is often heated during metal precursor reduction (to enable or accelerate the process). When incomplete metal precursor reduction takes place, the substrates with printed structures must often be further heated to enhance the electric conductivity by fully reducing the metal precursor films.
Direct laser writing (DLW), for instance, profits from the precise focusing of the irradiated precursor solution to photochemically reduce the metal precursor. Translating the laser focus in space can create 2D patterns and 3D structures as demonstrated, for instance, for Au [
34] and Ag [
35,
36]. Combined with photo-polymerization, the 3D structure is supported and mechanically strengthened by an as-generated polymer, which at high temperature post-treatment processes can be removed, leaving just the metal. However, without such shape-guiding polymers, simple metal pillars can be generated. A surfactant can be added to the precursor solution that acts as a growth inhibitor, enabling the fabrication of nanometric structures [
35]. Laser-induced reduction of a Ag precursor on poly(styrene-block-butadiene-block-styrene) also generated elastic patterns of Ag [
37].
To increase the rate of photochemical reduction without using a photocatalyst or heat, light irradiation can be combined with electrochemistry [
38]. While irradiating a metal precursor solution locally with a laser that is in contact with a conductive substrate, the substrate can be moderately polarized. The applied potential does not provoke direct electrochemical reduction of the metal precursor, which would cause homogeneous metal deposition over the entire polarized metal surface. Local metal patterning is enabled only where the laser irradiates the substrate. The combination of laser irradiation with electrode polarization enables the localized electron transfer from the substrate to the dissolved metal precursor for precursor reduction.
In an alternative approach, masks are used to accelerate the entire process by irradiating the complete area of interest for short periods without the need to translate components (e.g., printhead or light beam). Upon homogeneous irradiation of the entire mask, the mask blocks the light locally, while the non-blocking parts let the light pass for the local photochemical reduction of a metal precursor to M
0. Reduced pattern resolution could be caused by the diffraction of light, causing blurred instead of sharp pattern edges [
39].
To favor the photochemical reduction near the substrate surface for the deposition of metal NPs, the light exposure is often carried out from the bottom through the substrate, which can require that the substrate is transparent to the used wavelengths. Zhang et al. printed Ag and Au patterns photocatalytically using highly resolved UV irradiation of an Ag precursor salt solution that wetted a TiO
2-coated substrate [
40,
41]. The loading of Ag was controlled by the process parameters (e.g., light intensity, addition of PVP as stabilizer) as well as the type of substrate, enabling the printing of patterns of Ag gradients that were operated as plasmonic devices. The gold(I) thiourea complex ion Au(SC(NH
2)
2)
2+ was used instead of the tetrachloride gold ion complex AuCl
4− due to lower standard redox potential (
E°
[Au(SC(NH2)2)]2+/Au0 = +0.38 V vs. SHE instead of
E°
[AuCl4]−/Au0 = 1.00 V vs. SHE), which required the photocatalyst but enabled higher resolution during photocatalytic metal printing. Zuo et al. created M/MoS
2 particles (M was Ag and Pt) through photochemical reduction using a laser [
42]. Molybdenum is a semiconductor generating electron-hole pairs under irradiation. Pt/MoS
2 was studied as an electrocatalyst for the hydrogen evolution reaction (HER).
Yang et al. created patterns of citrate-stabilized Ag NPs by way of the photochemical reduction approach using image projection, which means the use of a so-called digital mask and a light projector [
43]. The limited electrical conductivity of the Ag patterns compared to bulk Ag had to be improved post-print, by treating the Ag patterns chemically with sodium chloride solution, which removed the organic capping agent that had reduced the inter-Ag NPs conductivity. The image projection approach has recently been further modified as polymer-assisted photochemical deposition [
44,
45]. Metal nanoparticles were synthesized in solution under UV-irradiation in the presence of a combined capping/reducing agent (citric acid) and immediately captured by a polymer (polyallylamine). Wu et al. reported the beneficial effect of adding anions to increase the conductivity of metal patterns obtained using the light projection approach [
46]. Wang et al. printed Ag, Au, and Pd patterns by projecting an image with a blue laser into a solution containing the metal cations [
47]. Their work showed that, after photochemical deposition with this approach, loose metal nanoparticles must be washed away from the substrate and additional thermal curing with the laser after printing can be necessary.
2. Print-Light-Synthesis of Mixed Metal Nanoparticles
Using inkjet printing for the deposition of metal precursor inks has another technical advantage apart from the precise loading of the precursor that can be achieved on the target substrate. By using different printheads in parallel, each filled with a precursor of a different metal, liquid mixed metal precursor films of well-defined ratios of the metals can be printed. This has also been realized with other ink-based material deposition systems but was usually followed by thermal treatments and not by high intensity light irradiation [
48,
49]. Parallel inkjet printing of different metal precursor inks in defined volume ratios on top of each other enables their controllable and adjustable mixture just before their exposure to high intensity light.
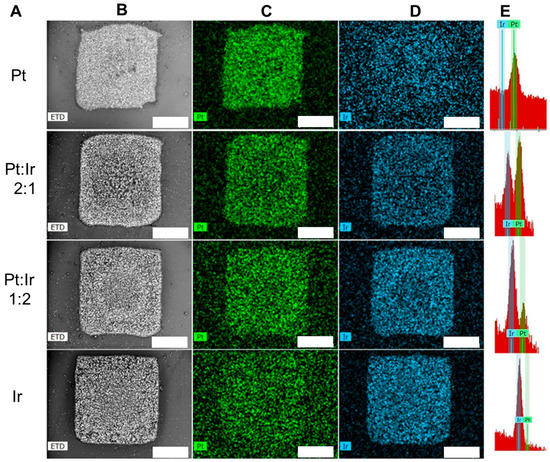
Figure 7 shows an example of mixed Pt and Ir patterns after Print-Light-Synthesis of mixed Pt
IVCl
62− and Ir
IVCl
62− inks using a high intensity Xe flash lamp. The total metal precursor loading was 3.0 μg mm
−2. Electron microscopy and spectroscopic analysis demonstrated the homogenous distribution of both metals in the patterns of 1 mm
2. Relative EDX intensities further demonstrate the relative ratios of both metal precursors.
Figure 7. Print-Light-Synthesis of mixed Pt and Ir patterns (3.0 μg total metal precursors weight mm−2) on ITO -coated glass slides with different ratios of the two metal precursor inks. Column (A): mass ratio between platinum and iridium precursor; (B) (greyscale): scanning electron micrographs of the patterns of mixed Pt and Ir after Print-Light-Synthesis; (C) (green): EDX maps of Pt; (D): EDX maps of Ir; (D) (blue): EDX maps of Pt; (E): EDX peaks for Ir and Pt. Scale bar 400 μm. Details can be found in the experimental section.
Print-Light-Synthesis is also applicable to non-noble metals. This was demonstrated, for instance, by Costa Bassetto et al. who fabricated thin electrode coatings of Ni
xFe
y nanoparticles that were synthesized on patterns of carbon nanotubes (CNTs) [
50]. The ratios of both metal precursors (here, FeCl
2 and NiCl
2 dissolved in an aqueous-based ink) were highly controllable by the printing parameters (or alternatively by the ink composition). Due to the low standard redox potentials of both metals (
E°
Fe2+/Fe0 = −0.41 V vs. SHE and
E°
Ni2+/Ni0 = −0.25 V vs. SHE), direct photochemical reduction of the metal precursors was not efficient with the process parameters and light source used. The process, therefore, required the presence of alcohols as reducing agents.
Examples other than patterning alloys or mixed metals are composites of metals with non-metals. Such composites are generally applied to increase the stability and activity of the metal nanomaterials. In many approaches, the metals are deposited on carbonaceous materials, such as carbon nanotubes and graphene [
51]. For instance, by using laser writing Zhou et al. fabricated patterns of a Cu/C composite. Besides an inorganic Cu
II precursor, tannic acid was added as carbon precursor to the ink that also contained reducing agents [
52]. The authors had the aim of enhancing the resistance of Cu to oxidation.
3. Oxidative Print-Light-Synthesis
The possibility to create oxidized metal species using Print-Light-Synthesis rather than metals of oxidation state zero is challenging, because the oxidation state of the metal after the process should not be zero and should potentially be even higher than it was in the precursor. One approach was recently realized by Silva et al., who developed an oxidative Print-Light-Synthesis approach to synthesizing Prussian Blue (PB) from ferrous (Fe
II) salts [
53]. Prussian blue and its analogues (PBAs) represent a promising group of materials that is currently considered a potential candidate for the cathode material in alkaline metal ion batteries. In PB, Fe
III4[Fe
II(CN)
6]
3 contains both Fe
2+ and Fe
3+. For the Print-Light-Synthesis of PB, a partial oxidation of Fe
II to Fe
III is, therefore, required. The hexacyanoferrate(II) anion ([Fe(CN)
6]
4−) was used as a Prussian Blue precursor.
The oxidative PLS was realized by placing the substrate with printed precursor ink patterns immediately into a gas chamber filled with oxygen and irradiating them with a Xenon flash lamp, in order to create an oxidizing atmosphere to potentially promote higher oxidation states of iron. However, the authors further described the necessary presence of inorganic acids, such as hydrochloric acid and sulfuric acid, to facilitate the redox processes. The authors suggested that the protons might act as electron acceptors, being therefore reduced to H2 (or forming HCN) and causing the partial oxidation of the FeII redox center (the only oxidation state of iron in the precursor salt) to Fe3+ (present in the obtained solid film together with FeII after Print-Light-Synthesis). The result was a blue deposit that the authors characterized electrochemically and spectroscopically as Prussian Blue.
Notably, under non-acidic conditions the partial oxidation of Fe
II to Fe
III was indeed not observed, confirming the assumption of the authors that protons must be present as electron acceptors. Electrochemical characterization of the as-obtained PB electrodes in acidic solution demonstrated the typical redox peaks for the oxidations and reductions of the two Fe redox centers in PB. Kindle et al. used direct laser writing to fabricate patterns of mixed metal oxides. In their approach, solvothermal synthesis for metal oxide preparation was combined with the patterning ability of the laser [
54]. Upon thermal decomposition of the precursors, metal oxides were formed. It must be noted, however, that this process worked only with non-noble metals (e.g., Fe, Ni and Cr) that easily generate an oxidized surface, while noble metals (e.g., Au, Pt and Ag) were fully reduced, generating M
0 patterns instead of patterns of metal oxides. McGee et al. fabricated multimetal (Cr, Fe, Co and Ni) bifunctional electrocatalysts in a similar way for overall water splitting (in particular, for oxygen evolution the oxides are more active than the metals with oxidation state zero) [
55]. Castonguay et al. printed mixed metal oxides of Cu, Ni, Zn, and Fe as gas sensing materials [
56], and Bae et al. fabricated ZnO and ITO with that approach [
57].
This entry is adapted from the peer-reviewed paper 10.3390/nano13131915