Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
The use of in vitro callus cultures as experimental model systems allows us to get closer to understanding the patterns and features of morphogenesis in intact plants. In this regard, the problem of realizing the morphogenetic potential of callus cells due to their pluri- and totipotency properties is of great interest. To solve this problem, it is important to use the histological approach, which involves studying the structures of developing tissues, organs and organisms in their interactions and relationships.
- plant morphogenesis
- histological analysis
- de novo organogenesis
- in vitro somatic embryogenesis
1. Introduction
Various morphogenesis pathways were revealed in calli in the subsequent stages of in vitro cultivation (Figure 1(4–11)): organogenesis (gemmogenesis-type or caulogenesis-type, consisting of the formation and development of the shoot; rhizogenesis-type, consisting of the formation and development of the root; or gemmorhizogenesis-type, consisting of the formation and development of the gemmorhizogenic structure that combines both the shoot and the root) and somatic embryogenesis (consisting of the formation and development of somatic embryos) (reviews [15,16,21,33]). Thus, reprogrammed callus cells exhibit the properties of pluripotency and totipotence under adequate in vitro conditions.
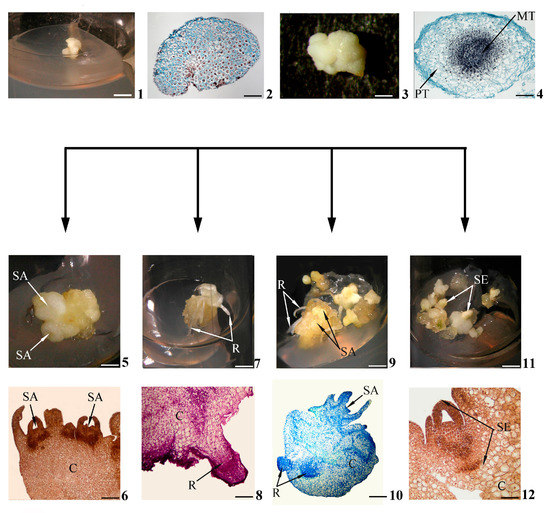
Figure 1. Stages of in vitro morphogenesis in calli. Primary morphogenic callus at initial stage of development according to morphological (1) and histological (2) data; developed primary morphogenic callus according to morphological (3) and histological (4) data; shoots (buds) in the morphogenic callus according to morphological (5) and histological (6) data; roots in the morphogenic callus according to morphological (7) and histological (8) data; gemmorhizogenic structures in the morphogenic callus according to morphological (9) and histological (10) data; somatic embryos in the morphogenic callus according to morphological (11) and histological (12) data. Symbols: C—callus, MT—meristematic tissue, PT—parenchymatic tissue, R—root, SA—shoot apex, SE—somatic embryo. Scale bars: (1) = 150 mm; (2) = 200 mkm; (3) = 3 mm; (4) = 100 mkm; (5,7,9,11) = 3 mm; (6) = 200 mkm; (8,12) = 100 mkm; (10) = 50 mkm.
2. Histological Approach to the Evaluation of In Vitro Morphogenesis Pathways in Calli
The results of studying the greatly important issue of plant regeneration in callus cultures in vitro have been summarized in a number of recent reviews.
Bidabadi and Jain [16], in their extensive review, evaluated the callus as a proliferating mass of dedifferentiated cells. Those authors analyzed in detail the physiological (mainly hormonal), biochemical, (epi)genetic and physical factors and some others that not only affect in vitro plant regeneration but also regulate this process. They paid much attention to the discussion of control molecular mechanisms, the occurrences of somaclonal changes and the role of programmed cell death, as well as many other issues connected to in vitro plant regeneration.
A cycle of works by Ikeuchi et al. [44,45,46,47,48,49] is devoted to various issues of callusogenesis, including a comparative analysis of events during plant regeneration in calli in planta and in vitro. Those researchers, assessing the callus as an unorganized mass of cells, considered the genetic and epigenetic mechanisms of regenerant formation. Those authors defined regeneration as a manifestation of either the reprogramming of differentiated somatic tissue cells or the activation of relatively undifferentiated somatic tissue cells. They paid special attention to the mechanisms of the molecular control of plant regeneration in calli formed in planta in response to wound exposure.
A number of reviews are devoted to the analysis of organogenesis events in calli. It should be noted that a number of authors use the phrase “in vitro organogenesis” when it comes to the formation and development of shoots with subsequent induction of root formation under the action of specific exogenous hormones. The other authors apply the phrase “de novo organogenesis” when they analyze the regeneration events of the shoots and roots in wound calli or in an isolated shoot or root. As evidenced by the analysis of the literature, the reviews of recent years have mainly been devoted to molecular events during organogenesis in calli. Thus, Zai and Xu [26], using the example of Arabidopsis thaliana, assessed the participation of the WOX5 gene in stimulating the acquisition of pluripotency properties by callus cells. Wang et al. [9] devoted their review to summarizing the achievements in the study of the molecular mechanisms of de novo organ regeneration in calli, especially the role of the WOX11 gene.
Researchers have paid great attention to in vitro somatic embryogenesis as a manner of plant regeneration in calli. In a theoretical study, Feher [24] posed the important problem of the correct interpretation of the terms “callus” and “somatic embryogenesis”, as well as the related terms “dedifferentiation” and “totipotence”. Sivanesan et al. [50], considering somatic embryogenesis to be a mode of stimulated plant cell totipotency, presented a review of the works devoted to the influences of major factors (nature of explant, abiotic stresses, concentration and variation of plant growth regulators) on the induction and regulation of somatic embryogenesis, including indirect somatic embryogenesis in callus cultures in vitro. Tretyakova and Mineev [51] analyzed data of indirect in vitro somatic embryogenesis in comparison with another system of asexual reproduction, which is apomixis. Those authors showed that the transitions of somatic cells to the totipotent and embryonic states occur at the physiological–biochemical and molecular genetics levels. A number of reviews have reflected the great interest of researchers in studying the influences of genetic (microRNA, transcription factors) and epigenetic (DNA methylation, chromatin remodeling) factors on the regulation of in vitro somatic embryogenesis events [52,53,54]. This direction of research is also demonstrated in a review by Rocha et al. [55]. Those authors analyzed the molecular mechanisms underlying the control of both morphogenetic pathways, de novo organogenesis and in vitro somatic embryogenesis, supported by findings involving proteome-, metabolome- and transcriptome-based profiles.
At the same time, there are relatively few studies in the literature specifically devoted to detailed histological analysis of in vitro morphogenesis pathways in calli. Nevertheless, certain histological patterns of these events have been identified.
Let us consider these histological events during in vitro gemmorhizogenesis, which is the most difficult and thus the most interesting morphogenesis pathway in calli. Schematically, this pathway is shown in Figure 1(1–4,9,10); more detailed histological data are given in Figure 2.
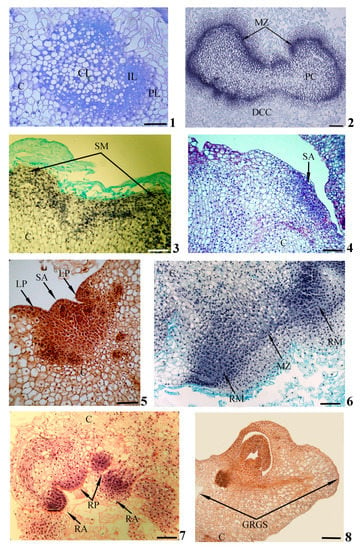
Figure 2. Histological events during in vitro gemmorhizogenesis in calli. Formation of zonality (1) and meristematic zone (2) within callus; formation of shoot meristem (3), shoot apex (4,5) and leaf primordia (5) on callus surface; formation of root meristems (6), root apex and root primordia (7) inside callus; formation of gemmorhizogenic structure (8). Symbols: C—callus, CL—central layer, DCC—destructive callus cell, GRGS—gemmorhizogenic structure, IL—intermediate layer, LP—leaf primordium, MZ—meristematic zone, PC—peripheral cell, PL—peripheral layer, RA—root apex, RM—root meristem, RP—root primordium, SA—shoot apex, SM—shoot meristem. Scale bars: (1,3–8) = 100 mkm, (2) = 200 mkm. Modified according to [29], with additions.
As stated above, a primary morphogenic callus at the initial stage of development (Figure 1(1)) consists of cells that are rather uniform in their sizes and structures (Figure 1(2)). Later (Figure 1(3)), a morphogenetic center is distinguished in the thickness of the callus. Such a center is represented by two cell zones, including the meristematic center as a central zone of densely located cells (Figure 1(4)).
In the process of development, the meristematic center, initially consisting of similar cells, will gradually acquire a distinct zonality. Some cells will remain meristematic, while others will become parenchymatic. Thus, the meristematic center consists of three cellular layers: a central layer presented by weakly vacuolated cells, an intermediate layer of meristematic cells and a peripheral layer of highly vacuolated parenchymatic cells (Figure 2(1)). In the course of subsequent development, the intermediate meristematic layer will increase in size due to the periclinal and anticlinal divisions of cells. This layer will be transformed into an expanded meristematic zone composed of several cellular layers and situated in parallel to the callus surface (Figure 2(2)). Simultaneously with the formation of the meristematic zone, the destruction of central and peripheral layers can be detected. In the upper callus area, where the complete destruction of the peripheral layer occurs, the shoot meristem (Figure 2(3)), the forming shoot apex (Figure 2(4)) and leaf primordia (Figure 2(4,5)) can be observed on the callus’ surface. Developing shoots have typical structures for plants in in planta conditions.
With a sufficient degree of shoot development, initiation of root meristems occurs in the part of the meristematic zone remaining inside the callus (as a rule, in the lower part of the callus below the developing shoot) (Figure 2(6)). The root apex and root primordia will gradually be formed inside the callus (Figure 2(7)). The developing roots are characterized by a structure that is typical for the roots of plants in in planta conditions.
As the shoots and roots develop, a connection will gradually be established between them by elements of the vascular system forming in the thickness of the callus. The united gemmorhizogenic structure will be formed (Figure 2(8)).
These analyzed histological events confirm the fact that the initial stages of indirect regeneration in calli are largely associated with the de novo formation of new meristems or the restructuring of previously existing meristems [2]. It has been shown, for example, that the initial realization of de novo organogenesis is closely related to the activity of cells in the superficial (epidermal, subepidermal) meristematic zones of Amorphophallus rivieri [35] and wheat [29] calli. Similar events were noted in somatic in vitro embryogenesis in calli (in [24]).
Important, in researchers' opinion, are the data on the participation of the differentiated surface cells of some organs in direct plant regeneration in vitro, without callus formation [56]. It should be noted that in natural conditions in planta, many initial morphogenetic processes, for example, the marking and laying of leaf primordia, will also occur in the meristem, namely in the peripheral zone of the apical meristem, functionally separated from the central zone and the meristem of expectation [57]. Above, researchers examined the similarities of histological events in primary morphogenic calli in vitro and root primordia in planta. All these examples may indicate the general mechanisms of regenerant formation in vitro and plant formation in planta.
In general, histological analysis has proven to be indispensable for the detailed study of events in calli during de novo organogenesis (namely gemmorhizogenesis) and in vitro somatic embryogenesis. Actually, the morphological features of the formed shoot and root are clearly distinguishable (Figure 1(5,7)), but it was very difficult to distinguish the gemmorhizogenic structure (Figure 1(9)) from the somatic embryo (Figure 1(11)) visually, especially in the late stages of its development. Only the histological callus investigation of the dynamics of development revealed that the gemmorhizogenic structure develops by alternately forming first the shoot on the callus surface and then the root in the callus thickness, followed by the gradual formation of the vascular system between these organs (Figure 2). This pattern of development distinguishes the gemmorhizogenic structure from the somatic embryo, in which the shoot and root are laid almost simultaneously [30]; compare also Figure 1(10,12).
Researchers also note that histological analysis alone did not confirm the formation of somatic embryos during in vitro direct regeneration in cultured petioles of Pelargonium × hortorum and Pelargonium × domesticum. It was revealed that leaf-like and shoot-like structures were formed in the conditions of the experiments performed [58].
It is important to emphasize that the data of detailed histological studies contribute to solving one of the fundamental problems of plant development biology, namely the similarities and differences between in vitro somatic embryogenesis and in planta zygotic embryogenesis (detailed more in [51,59,60]).
It is necessary to pay attention to the following aspect: Using the examples of many plants, it is well-established that the inductions of de novo organogenesis and in vitro somatic embryogenesis in calli are largely determined by an optimal balance of endogenous and exogenous phytohormones, and auxin and cytokinin play priority roles in determining callus cell fates [30,61,62,63,64,65]. To identify the mechanisms of endogenous phytohormone participation in these processes, an immunohistochemical method based on the histological approach was used. This method allowed obtaining of data of the contents and distribution of the hormones in plant cells (e.g., [66]). Thus, a comparison of the data of the immunohistochemical detections of endogenous auxin and cytokinin in the callus cells with the results of the histological analysis of the callus showed that, during de novo organogenesis, these hormones are localized mainly in the cells of actively developing organs—shoots and roots [29].
De novo organogenesis and in vitro somatic embryogenesis as systems of indirect regeneration are, as a rule, separately induced in calli. The leading exogenous factor of organ or embryo induction is the introduction of various hormones into the nutrient medium. At the same time, the literature has presented data from a few studies in which the processes of de novo organogenesis and in vitro somatic embryogenesis were revealed in calli using the identical hormonal composition of the nutrient medium. Thus, the formation of either shoots or somatic embryos on a medium of the same composition is morphologically described for the calli of Valeriana edulis ssp. procera [67], Metabriggsia ovalifolia W. T. Wang [68], Camellia nitidissima Chi. [69] and the Clematis sp. [70]. A number of works have provided data on the simultaneous formation of shoots and somatic embryos in the same callus on a medium of the same composition: for example, in Lavandula angustifolia Mill. [19], Stipagrostis pennata (Trin.) De Winter [32], Panax ginseng [71], Pogonatherum paniceum [72] and Scaevola sericea [73]. Researchers, as a rule, have not accompanied the presented morphological information with data that has histologically confirmed the formation of shoots and somatic embryos, especially in the dynamics of their development. In researchers' opinion, this once again indicates the need to use the histological approach, which allows the solving of many controversial issues that arise in studying the morphogenesis pathways in callus cultures in vitro. This is especially true for the pathways that lead to mass formation of the regenerants of economically important plants.
One of the possible ways to solve the problem of induction of both de novo organogenesis and in vitro somatic embryogenesis in the same callus is histological analysis of the positional location of the organ or embryo initial cells. The concept of positional information was proposed in order to understand the spatio-temporal organization of morphogenesis in the system of an integral organism [74]. This concept has been regarded ambiguously by researchers. Some authors have actively applied this concept in the analyses of various aspects of the developments of both plants [38,75,76] and animals [77]. Other authors have evaluated the concept as a formal, reductive mechanistic approach [78]. This issue should also be considered debatable. However, in researchers' opinion, the positive role of this concept in understanding the spatio-temporal organization of morphogenesis in calli is undeniable. In particular, the application of this concept can contribute to solving the questions of with which cells/groups of cells, in which place and in what specific form (organ or embryo) a new structure is formed in a callus as an integrated system.
Numerous experiments have been devoted to the investigation of various aspects of direct in vitro plant regeneration, without the stage of callus formation, as a rule, in response to certain stress treatments and/or exogenous hormone application. Recent reviews have been devoted to generalizing such experimental data. As evidenced by the analysis of the data presented in these reviews, in this case, plants also develop through de novo organogenesis or in vitro somatic embryogenesis. Studies over the last few years have expanded the understanding of the molecular mechanisms of cell reprogramming, and the results of genetic analysis have indicated the key role of epigenetic parameters in the regulation of direct in vitro plant regeneration [2,8,16,59,79,80,81]. In the context of researchers' review, data of the participation of differentiated surface cells of some organs in direct in vitro plant regeneration are important (see [57]), as well as data on changes in a number of histological parameters (for example, the area of the central cylinder and the cells of the primary root cortex) during direct salt-stress-induced in vitro rhizogenesis [82].
It is important to emphasize that histological events during direct in vitro regeneration largely coincide with similar events, not only during indirect regeneration in in vitro callus cultures but also in intact plants: for example, in case of wound exposure. Such data once again demonstrate the universality of plant morphogenesis events in vitro and in planta.
This entry is offline, you can click here to edit this entry!
