1. Introduction
Nanoparticle-mediated delivery for cancer immunotherapy can be categorized into two types based on their preparation: biologically synthesized nanoparticles and chemically synthesized nanoparticles. Biologically derived nanoparticles consist of biological components or cell derivative components, for example, exosomes and lipid-mediated nanoparticles, while chemically synthesized nanoparticles are categorized into polymers and inorganic nanoparticles (
Figure 1). The following section describes these nanoparticle-mediated delivery systems. Nucleic acid molecules are shielded by nanoparticle delivery systems [
67].
Figure 1. Nanoparticle-based platform for the delivery of nucleic acids.
2. Lipid Nanoparticles
Lipids are biological components that are the building blocks of the biological membrane [
68]. Liposomal nanoparticles have been approved by the FDA for clinical applications for cancer. Liposomal nanoparticles have been developed for site-targeted delivery (i.e., through conjugation with a targeting ligand) with a low toxicity and a high loading capacity due to the biological structure, tunable functionalization, and controlled release. They are thus attractive for use in delivering immune modulators. The properties and performance of lipid nanoparticles can be controlled by changes in the ratio of lipid constituents, surface chemistry, and method of synthesis; these variables influence the physicochemical nature of the lipid nanoparticles such as its size, shape, charge, and biological properties (i.e., the immune reaction, the efficiency of targeting, and the release of loaded ligands). Cationic lipid nanoparticles used in preclinical and clinical trials for gene and drug delivery often include amine head groups in the lipids [
69]. The lipid nanoparticles’ surface charge helps in the internalization of the cells due to the positive charge and activation of the immune response [
70]. pH-sensitive lipid nanoparticles have been used to enhance the targeted delivery of nanoparticles to subcellular compartments, particularly for the cytosolic delivery of immobilized immune modulators, leading to enhanced anticancer effects. The modification of the surface chemistry by cell-penetrating peptides (octaarginine and fusogenic peptides) has been described as an efficient strategy for inducing cross-presentation [
71]. These approaches are in the preclinical stage, demonstrating the need to continue development for clinical translation.
2.1. Lipid-Nanoparticle-Mediated circRNA Delivery
Accumulating evidence indicates the potential of circRNAs in the regulation of antitumor immune responses [
72,
73]. While circRNAs are under investigation as potential immunotherapeutic agents themselves, a major barrier to their application is the instability of circRNAs. Lipid nanoparticles can be used to encapsulate circRNAs to protect them from degradation, enhance cellular uptake and delivery via promoting the endosomal gateway for entry, and ultimately exert their antitumor function in vivo. CircRNA-loaded nanoparticles can be aimed at activating both innate and adaptive immune responses. For example, a novel ionizable lipid nanoparticle vaccine platform encapsulating the antigen-coding circRNA has been recently developed to exert the prolonged protein translation ability of circRNA and simultaneously create an innate immune stimulatory context, eventually leading to more potent cytotoxic antigen-specific T cell responses and a superior anti-tumor effect in multiple models of mouse tumors including “immune-desert” B16 orthotopic melanoma [
74]. An additional study has reported that intratumoral delivery of circular mRNA encoding a mixture of cytokines by lipid nanoparticles facilitated intratumoral and systematic antitumor immune responses and enhanced anti-programmed cell death protein 1 (PD-1) antibody therapy in a syngeneic mouse tumor model [
75]. Moreover, circRNA could serve as a potent adjuvant to trigger adaptive antitumor T cell immunity in the context of vaccination [
76]. Nevertheless, there is still a long way to go in the clinical translation of nanoparticle-based circRNA immunotherapy for various malignancies.
2.2. Lipid-Nanoparticle-Mediated mRNA Delivery
Many bioengineered nanoparticles are under development for delivering mRNA for immunotherapy, and some have advanced to clinical trials (e.g., the mRNA pancreatic cancer vaccine). Nanoparticles protect mRNA from nucleases and increase the internalization of mRNA into APCs, which increases the efficacy [
77]. Lipid nanoparticles consist of different types of lipids (cationic, anionic, and neutral). Lipid nanoparticles contain ionizable lipids, meaning that these lipids are positively charged at low pHs (enabling RNA complexation) and neutral at physiological pH; they merge with the cell membrane to internalize which bypasses lysosomal degradation [
78,
79,
80]. Nanoparticle uptake by the cell involves several highly regulated mechanisms such as phagocytosis, macropinocytosis, clathrin-mediated endocytosis, and caveolae, depending on physical properties of the nanoparticles such as size and shape, the chemical characteristics such as surface charge and modification, and the environmental conditions [
79,
81]. Cationic nanoparticles have been shown to efficiently deliver mRNA in vivo and elicit anti-tumor immune responses [
82]. Cationic lipid head groups have a positive charge, whose main functions are to provide electrostatic interactions between the lipids and the negatively charged nucleic acid and to control the interaction of the nanomaterial with the lipid membranes of target cells. Positively charged mRNA-encapsulated lipid nanoparticle vaccines stimulate cellular and humoral immune responses in both mice and humans, which leads to the synthesis of antigen-specific IgG antibodies and activation of antigen-specific T-cell responses for cancer immunotherapy [
83]. Moreover, the addition of polyethylene glycol (PEG) in lipid nanoparticles increases the stability and immobilization of nucleic acids but limits the uptake into cells [
84]. Clinical trials of mRNA-based vaccines have begun recently for patients with melanoma and squamous cell carcinoma. Lipid nanoparticles with mRNA aimed at inducing immune responses against the antigens NY-ESO-1, MAGE324, A3, tyrosinases, and TPTE are in clinical trials [
82]. Furthermore, lipid-nanoparticle-formulated nucleoside-modified mRNA vaccines encoding 20 patient-specific mutated neoepitopes are currently under clinical evaluation in combination with pembrolizumab in patients with solid cancers (NCT03313778 and NCT03897881). However, there are limitations to the number of currently clinically attempted antigens within mRNA, as true cancer-associated antigens encoding mRNAs represent only a very small fraction. The design space for mRNA has been expanded beyond therapeutic cancer vaccination using different nanoparticle formulations, delivery routes, and structural modifications. mRNA delivery by various nanoparticles is now employed for the most diverse immunotherapeutic approaches, including the equipping of immune cells with chimeric antigen receptors and the in vivo expression of immunomodulator proteins such as therapeutic antibodies, cytokines, or costimulatory molecules [
85].
2.3. Lipid-Nanoparticle-Mediated DNA Delivery
For the delivery of DNA, lipid nanoparticles are synthesized to prevent external hindrance. Delivery of plasmid-DNA-encoding costimulatory molecules and/or cytokines that stimulate tumor immunogenicity can be utilized efficiently by a nanoparticle because of its versatility. Indeed, a recent study has demonstrated the ability of a synthetic, biodegradable gene delivery nanoparticle platform to reprogram cancer cells in situ as tumor-associated antigen-presenting cells by inducing the coexpression of a costimulatory molecule (4-1BBL) and an immunostimulatory cytokine (IL-12) [
86]. Researchers synthesized a DNA-encapsulated liposomal vaccine which encodes for the melanoma antigen (MART1) [
87]. Liu et al. synthesized hydrophobic lipid nanoparticles with the help of DOPE for the delivery of CpG ODN along with the ovalbumin antigen. The acquired nanovaccines provide effective antigen stimulation which promotes the release of immune factors and leads to a boost in antitumor immunity to inhibit tumor growth [
88]. Numerous types of nanoparticle-based DNA delivery systems are under evaluation in both preclinical and clinical settings for cancer immunotherapy [
89,
90,
91].
3. Extracellular Vehicles
Extracellular vehicles (EV) are small particles which help in therapeutics and are generally synthesized by live cells. EVs are approximately 30 to 150 nm in size and found in different biological fluids [
92,
93]. Extracellular vehicles contain transmembrane markers, peptides, and specific proteins. EVs released from immune cells have unambiguous proteins and endosome-linked peptides, while EVs released from cancerous cells have an unambiguous tumor antigen. EVs are also now under investigation as delivery vehicles for immunotherapeutics. EVs are divided into immune-cell-derived extracellular vehicles and tumor-derived extracellular vehicles. The construction of synthetic EVs can address challenges associated with the need for sterility and scale manufacturing. Immune cells and dendritic cells derived from EVs have been reported to deliver MHC complexes such as MHC I and MCH II. CD80 and CD86 T cell stimulatory molecules and integrins act as adhesion molecules that additionally activate T cells [
94,
95].
Due to the unique tumor microenvironment and external treatment stimuli such as hypoxia and thermal stress, tumor-derived extracellular vesicles are typically more productive than immune-cell-derived extracellular vesicles [
96]. According to reports, these aggressively secreted substances contain unique tumor antigens that dendritic cells take up to trigger potent tumor-specific cytotoxic T lymphocyte responses [
97]. These extracellular functionalities of tumor derivatives have been used in several kinds of research to describe particular immunogenic responses for cancer immunotherapy. Additionally, nucleic acids or cytokines have been conjugated to extracellular vehicles to boost the tumor-derived extracellular vehicle’s efficiency in immunogenic activation, leading to the development of vaccines based on these extracellular vehicles. Future applications of tumor-derived extracellular vehicles as nanocarriers for cancer immunotherapy are hampered by the lack of clarity surrounding their precise impact and role on immunity. The therapeutic role of these extracellular vehicles for cancer immunotherapy needs to be assessed through research and safety evaluations.
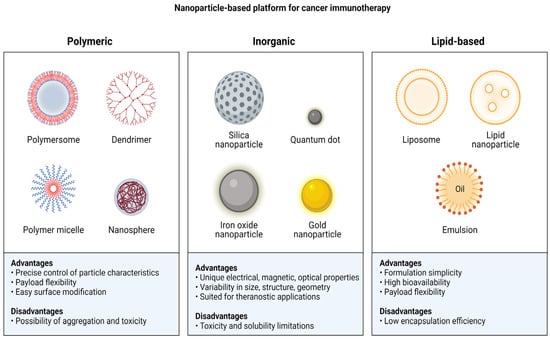
4. Polymeric Nanoparticles
Different types of polymers are widely used for the synthesis of polymeric nanoparticles which are used for cancer immunotherapy [
98]. These polymer-mediated nanoparticles can act as both adjuvants and delivery vehicles for immune-stimulating compounds when immune responses are stimulated to hinder the growth rate of tumors. Polymer-mediated nanoparticles are widely used as adjuvants in cancer immunotherapy due to their superior biocompatibility and solubility and high loading efficiency for immune-coupled components. Surprisingly, certain useful polymer-mediated nanoparticles are capable of stimulating immune responses against tumor cells. Polymer nanoparticles consisting of a cationic and responsive polymer such as poly (b-amino ester) have been examined to load ionic mRNA via an electrostatic interface, and these nanoparticles show enhanced transfection effectiveness and the curative effects of mRNA in vitro [
56]. A bioactive polymer called inulin acetate (InAc) was used in conjunction with a poly lactic-co-glycolic acid (PLGA) as an associated delivery platform that could serve as a pathogen-incorporated vaccine delivery system [
99]. The most frequent choice for polymeric nanoparticles in cancer immunotherapy is the PLGA nanoparticle [
100]. PLGA nanoparticles have the self-capability to target dendritic cells and APCs. PLGA nanoparticles are also able to attach to tumor antigens and adjuvants. These biodegradable and biocompatible PLGA nanoparticles have been approved by FDA for delivery applications. Moreover, in vivo, research revealed that PLGA-loaded nanoparticles moved to the lymph nodes, where they activated dendritic cells and boosted CTL responses, which improved the efficacy of melanoma, bladder, and renal cell carcinoma. To transport more antigens to the targeted region and improve DC targeting, surface modification of PLGA nanoparticles was investigated. Although PLGA nanoparticles can increase immunity in preclinical models, their clinical performance still must be enhanced. Typically, an amphiphilic polymer’s hydrophobic moieties constitute the inner core of the structure, while hydrophilic residues form the outer covering [
101]. Alternative nanocarrier substrates are required since many strong immune-stimulating drugs are protein based.
Hydrogel-mediated nanoparticles are particularly appealing for delivering hydrophobic compounds because of their exceptional capacity for hydrophobic molecules. Unfortunately, there are problems with current nano hydro gel platforms, especially concerning their unpredictable release characteristics. The physiological setting and inherent qualities of hydro gels prevent their further use in clinical research due to rupture release and short-term delivery caused by the expanded pore size and rapid degradation [
102]. To enable construction with reliable and sustained delivery, improvements to better manage the cargo release from hydrogels are required.
5. Inorganic Nanoparticles
Cancer immunotherapy can make extensive use of inorganic nanoparticles by way of the precise control over the size, charge, and surface modifications [
103]. They offer great efficiency and reproducibility for cancer vaccination techniques [
104]. Inorganic nanoparticles have distinctive optical features that can be used for controlled tumor ablation with immunotherapy using nanoparticles [
105].
Of all the inorganic nanoparticles, gold nanoparticles (AuNPs) have undergone extensive research for tumor immunotherapy. They are excellent delivery candidates to deliver tumor antigens and immune adjuvants to the targeted site due to their compliant surface chemistry and customizable shape [
106]. Interaction of AuNPs with dendritic cells leads to triggered immune stimulant cytokine expression and downregulates immunosuppressive chemokines [
107]. The elevated phagocytic features of dendritic cells and the improved maturation and triggering of T cell-linked immunological reactions are signs that AuNPs trigger dendritic cell activation [
108]. AuNPs can serve as adjuvants when conjugated with CpG ODN (and other nucleic acids for immune response), in addition to their capacity as carriers of antigens to dendritic cells. Additionally, AuNPs can attract near-infrared radiation due to their unique optical properties, which generates heat for tumor ablation, a treatment method known as photothermal therapy. AuNPs induce tumor cell apoptosis and necrosis using photothermal therapy and appear to release tumor antigens and intrinsic immune adjuvants such as heat shock proteins (HSPs), which significantly stimulated immune activation [
109].
Other metallic nanocomposites, such as silver and iron, have demonstrated tremendous activity against cancer immunotherapy in addition to the well-established AuNP systems. Metallic nanoparticles (MNPs) are used for therapeutic purposes in ongoing or finished clinical trials due to the above-mentioned intrinsic benefits. A well-known nanomedicine called CYT-6091 has been investigated in early clinical trials for cancer immunotherapy. It is made of AuNPs functionalized with thiolated PEG and recombinant human tumor necrosis factor-a (rhTNF-a) [
110]. Advanced-stage cancer patients participated in phase I dosage escalation trials testing CYT-6091. According to the results, rhTNF formulated as CYT-6091 was given systemically, resulting in precise tumor targeting with no side effects.
Even with MNPs’ examination in ongoing clinical phase trials, their potential clinical applicability is debatable, mostly due to safety concerns such as the accumulation of AuNPs over time in the organs. After 10 months of research in dogs, AuNPs were found to be toxic; the results revealed pigmentation and accumulation in the liver, spleen, and lymph nodes [
111]. In addition, the oxidation of DNA, lipids, and protein in cells is caused by ROS and free radical generation by AuNPs. This could change cell functions and even result in cell death [
112].
6. Hybrid Nanoparticles
Hybrid nanoparticles can consist of both inorganic (e.g., metal ions, metal clusters, or particles, salts, oxides, sulfides, non-metallic elements, and their derivatives) and organic (e.g., organic groups or molecules, ligands, biomolecules, pharmaceutical substances, polymers) components to exploit the advantage of both components to enhance their biocompatibility and efficiency and reduce their toxicity. Hybrid nanoparticle synthesis is based on two strategies: barge and tanker. According to barge and tanker strategies, liposomes, micelles, polymers, and noble metals are encapsulated (barge) or accumulated at the surface (tanker) of nanoparticles [
113]. Magnetic gold multifunctional hybrid nanoparticles have been developed, which consist of a gold nanoshell with a superparamagnetic iron oxide silica core, allowing MR imaging as well as photothermal therapy [
114]. Efficient eradication of primary tumors and metastasis in mouse models can be achieved using PLGA nanoparticles co-encapsulated with hollow gold nanoshells (HAuNS, a photothermal agent) and an anti-PD-1 peptide (AUNP12) by blocking the PD-1/PD-L1 pathway and potentiating antitumor T cell responses [
115]. Additional strategies include hybrid nanoparticles synthesized for the delivery of mRNA by using cationic lipids (e.g., DOTAP) and cationic biopolymers (e.g., protamine), which increases the transfection efficacy compared to lipid-mediated mRNA delivery and polymer-mediated mRNA delivery alone. Another hybrid nanoparticle strategy is dendrimer-mediated lipid nanoparticles, which consist of PEGylated BODIPY dyes that combine mRNA delivery and NIR imaging [
116]. There are many other examples of hybrid nanoparticles, including PLGA lipid nanoparticles loaded with CRISPER/Cas9 plasmids, which consist of PLGA, lecithin, DSPE-PEG-cRGD, and DSPE-PEG-biotin [
117].
7. Spherical Nucleic Acids (SNAs)
Spherical nucleic acids (SNA) are another class of smart nanoparticles that are synthesized by chemically modifying spherical cores with dense layers of single- or double-stranded DNA or RNA [
118]. SNAs have generated a great deal of interest since Mirkin and colleagues initially reported them in 1996 because they enable a new approach for gene and medication delivery. The distribution of nucleic-acid-mediated therapeutics and the biological activity of the nucleic acid presented by the nanoparticle are enhanced. Recent developments in SNAs have accelerated both their potential for usage in clinical settings and their biomedical applications.
7.1. Selection of an Appropriate Core for SNAs
Selecting an appropriate nanoparticle core is crucial for the effective delivery of nucleic acids utilizing SNAs. The forms, sizes, and biological profiles of the core–shell assembly are directly influenced by this. For organized DNA/RNA grafting on their surfaces, inorganic nanomaterials have been employed, such as iron, gold, silver, Pd, and Pt nanoparticles [
119,
120,
121]. To create more biocompatible SNAs, organic nanomaterials such as proteins, polymers, and liposomes have been used [
83,
122,
123,
124]. The most popular SNA core materials are covered in this section, along with an explanation of how they were developed for dense nucleic acid coverings.
Inorganic Cores for SNA
Gold nanoparticles (AuNPs), which are the most characteristic noble metal nanoparticles, have been extensively used as the primary material in SNA synthesis. The size and form of the final SNA products can be directly controlled by producing AuNPs using simple synthetic procedures to generate a wide variety of particle diameters [
125]. To create gold core SNAs, 13 nm AuNPs were capped with thiol-DNA in 1996. This process allowed for colloidal aggregation to occur in a controlled, thermally reversible mode [
119]. The dense coating of DNA influenced the optical, electrical, and biological properties [
126]. As a result of the surface area of AuNPs, multifunctional components can be presented densely, facilitating advanced drug/gene delivery for therapeutic applications. Silver nanoparticles (AgNP), in addition to gold, make excellent candidates for DNA grafting. Magnetic resonance imaging (MRI) and magnetic structure construction for electronic memory are just a few of the numerous uses enabled by densely functionalized iron oxide nanocrystals (Fe
3O
4 nanoparticles) with a surface DNA coating [
127,
128]. Metallic nanoparticles with hydrophobic capping agents, such as platinum, aluminum, palladium, copper, cobalt, and their combinations, can be used to make more forms of SNAs [
129].
Organic Cores for SNA
Even though inorganic nanoparticles are most frequently employed to make SNAs, their propensity to concentrate in organs such as the liver and spleen poses possible long-term health complications [
105,
130]. ODNs are frequently washed out of cells, whereas the nanoparticle cores remain inside once SNAs are internalized by endosomes during incubation [
131]. As a result, recently created biodegradable as well as biocompatible nanoparticles, including proteins, liposomes nanoparticles, and poly(lactic-co-glycolic acid) (PLGA), have created intriguing new opportunities for SNA functions in the biomedical field.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15061743