The term “gynecologic cancer” defines any cancer that arises in a woman’s reproductive system. During the period 2012–2016, 94,000 women were diagnosed with gynecologic cancer annually [
1,
2]. With an incidence of more than 3.6 million per year and mortality exceeding 1.3 million per year, these cancers constitute a public health issue, accounting for 40% of all cancer incidences and more than 30% of all cancer deaths in women worldwide [
3,
4]. Between 1990 and 2017, the age-standardized prevalence and incidence rate of EC increased globally by 0.89 percent and 0.58 percent a year, respectively, with a median age of diagnosis of 65 years [
5,
6,
7]. According to recent statistics, incidence of EC is estimated to be 15% for patients ≤ 50 years old and 4–14% for those ≤40 years old [
8]. For the year 2020, the World Health Organization estimated an EC incidence of 35,915 cases in women ≤ 44 years, 14,203 in women ≤ 39 years, and 2232 in women ≤ 29 years of age [
9]. The Western lifestyle, with the associated spread of clustered risk factors such as excess weight, diabetes mellitus, hypertension, and high serum triglycerides, contributes to the emergence of EC [
10]. PCOS, dysfunctional uterine bleeding/anovulation, and hypermenorrhea are other emerging risk factors linked to EC [
9]. EC should be a trending topic for public health professionals: the IARC estimated that EC cases will increase by more than 50% worldwide by 2040 [
5,
11]. Different risk factors are associated with Type I and II EC, and partially mirror those of women’s risk for other cancers [
2,
12,
13,
14]. The main risk factor for type I EC is prolonged exposure to excess estrogen without adequate balance by progestin [
15,
16]. Sources of exogenous estrogen include hormone replacement therapy and Tamoxifen, while endogenous estrogen exposure may result from high body weight, dysfunctional menstrual cycles, or, in rare cases, tumors that secrete estrogen. Unopposed systemic estrogen therapy results in a high risk of endometrial hyperplasia (20–50% of women) [
17] with a relative risk of EC ranging from 1.1 to 15 [
18]. The use of the selective estrogen receptor modulator Tamoxifen increases the risk of EC in women after menopause with an effect that is duration- and dose-dependent with a RR of 3.32, and 95% CI 1.95–5.67 [
19]. The dietary supplementation of phytoestrogens (non-steroidal chemical products with estrogenic and anti-estrogenic properties) for a time longer than 12 months may potentially increase the risk of EC (3.8%) [
20]. The most common disorder associated with anovulation is polycystic ovary syndrome. Anovulatory women have an imbalance of sex hormones that leads to irregular uterine bleeding and continued proliferation of the endometrium that can lead to endometrial hyperplasia. Obese women have high endogenous estrogen levels and endocrine abnormalities such as altered levels of insulin-like growth factor and insulin resistance. Early menarche and late menopause increase the risk of the disease. Some ovarian cancers that produce estrogen, such as granulosa cell tumors, are most likely associated with endometrial neoplasia (25–50 per cent of women affected) and carcinoma (5–10 per cent of the affected) [
21]. Some genetic syndromes are decisive risk factors for EC; Lynch syndrome, for example, accounts for 2–5% of all EC [
22], and BRCA gene mutation significantly increases uterine cancer (RR 2.65, 95% CI 1.69–4.16) [
23]. The risk of EC is significantly elevated, especially for BRCA mutation carriers taking the drug Tamoxifen [
24]. Some risk factors that are associated with EC include nulliparity, infertility, hypertension, and diabetes [
25]. Protective factors for type I EC include combined estrogen–progestin oral contraceptive use (decreases endometrial carcinoma risk by 30 per cent or higher [
26]), childbearing at an older age, and breastfeeding. Cigarette smoking is associated with a diminished risk of EC in postmenopausal women relative risk (RR 0.71, 95% CI 0.65–0.78) [
27]. Increased physical activity appears to reduce the risk of EC (RR 0.80, 95% CI 0.75–0.85) [
28]. The habit of drinking coffee decreases the risk of EC with a dose-dependent rate. The reductions in risk for low/moderate drinkers were RR 0.87 (95% CI 0.78–0.97), and for heavy coffee drinkers were RR 0.64 (95% CI 0.48–0.86) [
29]. Additionally, tea consumers have a decreased risk of EC proportional to the quantity consumed, especially for green tea (RR 0.8, 95% CI 0.7–0.9) [
30]. Type II endometrial neoplasms have different risk factors than type I EC and are less well known because of their rarity. Obesity is less strongly correlated [
31]. Pluriparity is a risk factor [
32]. Type II tumors have a different racial distribution; type II EC is more common in Black women than in White women [
33].
2. Diagnostic Work Up
Abnormal uterine bleeding (AUB) and heavy menstrual bleeding (HMB) are common conditions affecting 19.5% of women of reproductive age [
66]. In 2011, FIGO provided definitions of AUB, HMB, and Chronic AUB that should be used in medical literature and current clinical practice to standardize language [
67]. The acronym PALM-COEIN could facilitate accurate diagnosis and treatment of uterine bleeding: PALM stands for the causes that can be assessed by imaging and pathology, such as polyps, adenomyosis, leiomyoma and malignancy, while the word COEIN stands for non-structural causes such as coagulation disease, ovulation problems, endometrial causes, iatrogenic, and others causes [
68]. Abnormal bleeding is one of the main symptoms of all types of uterine disease, but has low specificity for malignancy. There is no correlation between AUB and the FIGO stage of the tumor; in addition, women not presenting AUB at the diagnosis of EC showed significantly better prognosis [
69]. Bleeding disorders such as abnormal premenopausal and postmenopausal bleeding are the main EC symptoms for which patients seek gynecological consultation. Transvaginal ultrasonography is a safe, straightforward, and easy way to examine double-layered endometrial thickness and to triage women for further investigations [
70]. According to international guidelines, gynecologists should assess endometrial thickness with transvaginal ultrasonography (TVUS) in women with abnormal bleeding that arises after menopause [
71,
72]. A thin endometrium should reassure clinicians about EC and lead to expectation management with seriated TVUS. In cases with a thickened endometrium, endometrial biopsy is warranted. TVUS diagnostic accuracy for EC diagnosis depends on the cut-off in use. The British Gynaecological Cancer Society guidelines currently recommend an endometrial thickness cut-off of ≥4 mm that has shown 94.8% sensitivity, 46.7% specificity, and a 99% negative predictive value for EC detection [
70,
71]. There is not consensus on the best cut-off to use to select AUB patients requiring endometrial biopsy; there is a high prevalence of EC in symptomatic patients when TVUS showed a thickness < 4 mm (8.5%). Some authors have suggested new diagnostic tools for the assessment of EC [
73]. One of the main prognostic factors of EC is the depth of myometrial invasion [
74], which strongly correlates with the 5-year survival rate: 94% for EC confined to the endometrium, 91% for EC in the inner 1/3 of the myometrium, and 59% when EC is in the outer 1/3 of the myometrium [
75]. In addition, myometrial invasion correlates with the risk of extrauterine extension of EC: tumors confined to the inner 1/3 of the myometrium have a 12% risk of extrauterine extension while tumors invading the outer 1/3 have a 46% risk [
76]. Contrast-enhanced magnetic resonance imaging (MRI), prevalently T1-weighted imaging including DCE MRI, is the most accurate diagnostic tool for the deep myometrium, with a sensitivity of 72–94% and a specificity of 87–96% [
77,
78,
79,
80]. Because of the high cost and technical issues related to MRI, physical examination followed by office vaginal ultrasound is a more affordable and accessible diagnostic technique proposed for deep myometrial invasion assessment, with an estimated sensitivity of 75% and specificity of 86% [
81]. Biopsy provides the definitive diagnosis: hysteroscopy-guided biopsy remains the gold standard for diagnostic EC, (sensitivity 99.2%, specificity of 86.4%) [
82]. Tao brush cytology and Pipelle have a positive predictive value of 81.7% and negative predictive value of 99.1%, but have sampling issues [
83]. Blind dilatation and curettage has the highest undiagnosed rate for EC. Hysteroscopy with directed biopsy/curettage is more effective in diagnosing cervical involvement (specificity 98.71% vs. 93.76% (
p < 0.01)) and more accurate in diagnosis of EC histology type and tumor grade than blind D and C. In
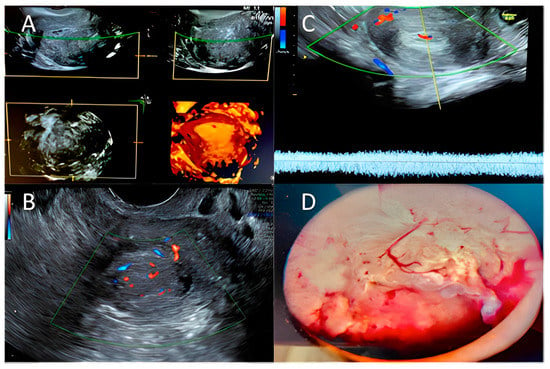
Figure 1 we summarized the main ultrasonographic and hysteroscopic findings in endometrial hyperplasia and endometrial cancer.
Figure 1. Hyperplasia/endometrial cancer: (A) 3D ultrasound exam; (B,C) Color–Doppler score examination—Courtesy of MD and VP; (D) Hysteroscopic typical pattern for endometrial hyperplasia/endometrial cancer. Courtesy of GRD.
3. Conventional Treatment: NCCN Guidelines
The standard management of EC involves surgery, chemotherapy, and/or radiation therapy. The gold standard staging procedure for EC is total hysterectomy with bilateral sal-pingo-ovariectomy (TH/BSO) with, if necessary, lymph node surgical assessment [
90]. In some selected premenopausal patients, ovary preservation may be a safe choice in stage I endometrioid cancer [
91]. Minimally invasive surgery does not compromise oncological outcomes and has a lower rate of complications, so should be proposed in patients with macroscopically uterine-confined cancer. A LAP2 trial compared oncological outcomes in laparoscopic vs. laparotomic surgery, showing recurrence rates of 11.4% for LPS versus 10.2% for LPT surgery and a 5-year overall survival rate of up to 84.8% [
92,
93]. A trial by Maurits et al. [
93] showed a significant complication rate of 14.6% in laparoscopy versus 14.9% in laparotomy, and a minor complication rate of 13.0% in laparoscopy versus 11.7% in laparotomy. Laparotomy remains the gold standard for patients with old age, a large uterus, or metastatic presentations [
94]. Robotic surgery may be the surgical choice for the severely obese and for patients at higher anesthesiologic risk [
95]. During the surgery, suspicious intraperitoneal areas and enlarged lymph nodes should be biopsied and peritoneal cytology should be collected. Through surgical staging, an accurate diagnosis, extension of the disease, a prognostic assessment and patients who require further adjuvant therapy can be defined. Routine lymph node dissection identifies patients with nodal localization requiring adjuvant treatment with radio and/or chemotherapy [
96,
97,
98]. Guidelines recommend sentinel lymph node biopsy in patients with low-risk and intermediate-risk diseases. Radiotherapy plus brachytherapy, external beam radiation, and the combination of both, or chemotherapy with carboplatin with a given area under the free carboplatin plasma concentration versus time curve of 5–6 plus paclitaxel 175 mg/m
2 are the standard adjuvant therapies that are proven to lower the risk of tumor recurrence. Adjuvant treatment recommendations for EC strongly depend on the prognostic risk group. For low-risk ECs, no adjuvant treatment is recommended [
86]. In intermediate-risk populations, adjuvant brachytherapy should be proposed [
86]. Adjuvant chemotherapy should be proposed in high-risk populations, especially for high grade and/or substantial LVSI. The omission of adjuvant treatment should be considered if a close follow-up is guaranteed. In stages III, IV, and recurrent EC, debulking surgery should be performed only if complete macroscopic resection is possible with acceptable morbidity. Primary chemotherapy should be used if debulking surgery is not feasible or acceptable [
99].
Immunotherapy as a New Approach in EC
One of the fields of interest in gynecological carcinomas is immunotherapy. The basic principle is that cancer grows when the host’s immune system is abnormal, and immunotherapy strengthens the patient’s immune system, allowing it to act better against cancer cells, slowing the growth and inhibiting the spread of the cancer [
100]. Therefore, as in other female solid carcinomas [
101], the evaluation of tumor infiltrating lymphocytes (TILs) has a fundamental role in predicting the response to immunotherapy. Currently, there are several immunotherapy strategies, among which those related to programmed cell death protein 1 (PD-1) and its ligand (PDL-1) are very encouraging. PD-1 and PDL-1 are proteins that inhibit the T-lymphocyte-mediated inflammatory response and allow cancer to evade apoptosis. PD-1 is a transmembrane protein expressed on the surface of lymphocytes which acts as an immunological checkpoint, i.e., it prevents the excessive activation of immune system cells from which immune and autoimmune responses arise [
102]. Using anti-PD1 or PDL-1 molecules, immunotherapy inhibits the immune inhibitory system and consequently activates the patient’s immune system against cancer [
103,
104]. In a study of 437 ovarian and endometrial solid tumors, PD-1 expression was found in 80% to 90% of cases [
105]. An example is Pembrolizumab, which is used as a promising therapy in carcinomas showing loss of MMR proteins such as melanoma and endometrial cancer [
106,
107,
108]. Loss of function of the phosphatase tumor suppressor PTEN, which blocks the PI3K/AKT/mTOR pathway, is another area of research. The use of Temsirolimus, an mTOR inhibitor, was studied in a phase II study of 62 patients with recurrent metastatic endometrial cancer and demonstrated a remarkable response in patients who had not yet received any chemotherapy, regardless of PTEN status [
109]. Unfortunately, in another phase II study of 42 patients with platinum-resistant ovarian cancer and advanced endometrial cancer, Temsirolimus treatment failed and the study was suspended [
110]. Furthermore, patients with high microsatellite instability (MSI-high) also have a better response to immunotherapy. This is probably due to the fact that the excessive mutational load leads to an elevated expression of neo-antigens by each TILs-recalling cell, resulting in a response to immunotherapeutic drugs [
111]. In May 2017, the US Food and Drugs Administration (FDA) accelerated the use of Pembrolizumab in patients with MSI-high or MMR protein loss in solid tumors. This was the first time the FDA has approved a treatment for patients with a specific molecular signature and not based on the location of the primary tumor. This decision was also supported by clinical studies in other cancer histotypes (NSCLC, melanoma, colon), which demonstrated that patients with high MSI molecular labeling or MMR protein deficiency had a very marked improvement in response outcomes [
112,
113,
114,
115]. These studies have been foundational and are leading to the validation of immunotherapy in endometrial cancer [
116]. In 2017, the phase 1b study KEYNOTE-028 on the effect of Pembrolizumab on advanced or metastatic CE with PDL-1 positivity already treated with standard therapy showed a partial response in three patients, of which one had a mutation in POLE. The cumulative response was 13% with a six-month PFS of 19% and overall survival of 68.8%. Only mild adverse effects were found in 54.2% of patients [
117]. Furthermore, in a recent phase Ib/II study, the combination of Pembrolizumab and the TKI Lenvatinib was tested in 23 patients with progressive metastatic EC, after standard chemotherapy. This study saw a 48% cumulative response with mild adverse effects [
118]. Finally, there are numerous active clinical studies in the field of immunotherapy from which we expect promising answers in such a way as to be able to identify specific cohorts of patients also on the basis of molecular characteristics and genetic signatures.