The glycocalyx generally covers almost all cellular surfaces, where it participates in mediating cell-surface interactions with the extracellular matrix as well as with intracellular signaling molecules. The endothelial glycocalyx that covers the luminal surface mediates the interactions of endothelial cells with materials flowing in the circulating blood, including blood cells. Cardiovascular diseases (CVD) remain a major cause of morbidity and mortality around the world. The cardiovascular risk factors start by causing endothelial cell dysfunction associated with destruction or irregular maintenance of the glycocalyx, which may culminate into a full-blown cardiovascular disease. The endothelial glycocalyx plays a crucial role in shielding the cell from excessive exposure and absorption of excessive salt, which can potentially cause damage to the endothelial cells and underlying tissues of the blood vessels.
- endothelial-glycocalyx
- proteoglycans
- syndecans
- hyaluronan
- glypicans
- sodium chloride-salt
- CVD
1. Background
2. Major Components of the Glycocalyx in CVD Pathophysiology
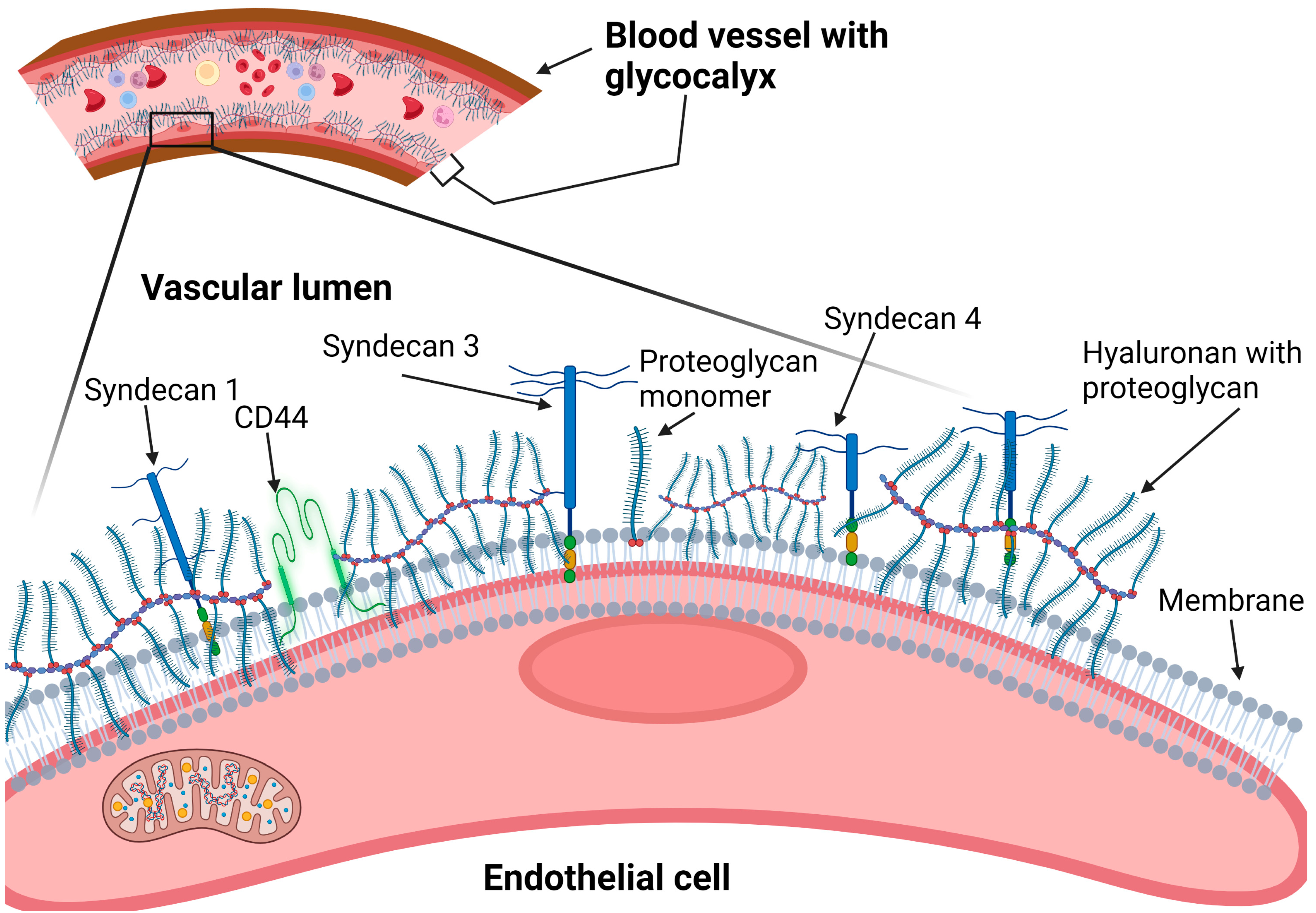
2.1. Heparan Sulfate Proteoglycans (Syndecans and Glypicans)
2.1.1. Syndecans
2.1.2. Glypicans
2.2. Hyaluronan
3. Glycocalyx and Salt Interactions

3.1. Proposed Glycocalyx-Salt Interaction Mechanisms Contributing to Hypertension and Cardiovascular Disease
3.2. Possible Strategies for Reducing the Damaging Effects of NaCl-Salt Overload on Vascular Endothelium
High dietary salt intake remains a big challenge as many people in various populations around the world are still unable to stop consuming high amounts of salt due to the diverse sources of dietary salt available in common foods [80][81][82]. It is therefore understandable that efforts are being made to find alternative ways of overcoming the deleterious effects of high salt overload on human health, other than simply advising people to reduce salt intake. For example, a recent clinical trial conducted among black women aged between 20 and 60 years in the USA provided evidence showing that ‘hot yoga’ can reduce the harmful effects of salt overload on endothelial function [83]. The exact mechanism is unknown. Similarly, regular aerobic exercise has also been reported to reduce endothelin-1-mediated vasoconstriction and endothelial dysfunction in postmenopausal women, as well as in obese or overweight adults [84][85]. Replacement or substitution of NaCl salt with other forms of salt with similar ‘saltness’ taste, such as potassium chloride (KCl) and monosodium glutamate (MSG), have also been piloted by the Department of Food Science at Cornell University (New York) and been found to have relatively high acceptability among the study subjects, although with a caveat of not disclosing the specific names of the salt substitute [86].
4. Current Diagnostic Tools to Determine Glycocalyx and Endothelial Health Status
5. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/nu15132873
References
- Mandrycky, C.J.; Howard, C.C.; Rayner, S.G.; Shin, Y.J.; Zheng, Y. Organ-on-a-chip systems for vascular biology. J. Mol. Cell. Cardiol. 2021, 159, 1–13.
- Daly, C.J. Examining Vascular Structure and Function Using Confocal Microscopy and 3D Imaging Techniques. Adv. Exp. Med. Biol. 2019, 1120, 97–106.
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329.
- Clough, G. Relationship between microvascular permeability and ultrastructure. Prog. Biophys. Mol. Biol. 1991, 55, 47–69.
- Stan, R.V.; Tse, D.; Deharvengt, S.J.; Smits, N.C.; Xu, Y.; Luciano, M.R.; McGarry, C.L.; Buitendijk, M.; Nemani, K.V.; Elgueta, R.; et al. The diaphragms of fenestrated endothelia: Gatekeepers of vascular permeability and blood composition. Dev. Cell 2012, 23, 1203–1218.
- Dull, R.O.; Hahn, R.G. The glycocalyx as a permeability barrier: Basic science and clinical evidence. Crit. Care 2022, 26, 273.
- Jin, J.; Fang, F.; Gao, W.; Chen, H.; Wen, J.; Wen, X.; Chen, J. The Structure and Function of the Glycocalyx and Its Connection with Blood-Brain Barrier. Front. Cell. Neurosci. 2021, 15, 739699.
- Haymet, A.B.; Bartnikowski, N.; Wood, E.S.; Vallely, M.P.; McBride, A.; Yacoub, S.; Biering, S.B.; Harris, E.; Suen, J.Y.; Fraser, J.F. Studying the Endothelial Glycocalyx in vitro: What Is Missing? Front. Cardiovasc. Med. 2021, 8, 647086.
- Wang, G.; Tiemeier, G.L.; van den Berg, B.M.; Rabelink, T.J. Endothelial Glycocalyx Hyaluronan: Regulation and Role in Prevention of Diabetic Complications. Am. J. Pathol. 2020, 190, 781–790.
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952.
- Melrose, J. Perlecan, a modular instructive proteoglycan with diverse functional properties. Int. J. Biochem. Cell Biol. 2020, 128, 105849.
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; oude Egbrink, M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflug. Arch. 2007, 454, 345–359.
- Oberleithner, H. Vascular endothelium: A vulnerable transit zone for mercilesssodium. Nephrol. Dial. Transplant. 2014, 29, 240–246.
- Bkaily, G.; Jacques, D. Morphological and Functional Remodeling of Vascular Endothelium in Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 1998.
- Knežević, D.; Ćurko-Cofek, B.; Batinac, T.; Laškarin, G.; Rakić, M.; Šoštarič, M.; Zdravković, M.; Šustić, A.; Sotošek, V.; Batičić, L. Endothelial Dysfunction in Patients Undergoing Cardiac Surgery: A Narrative Review and Clinical Implications. J. Cardiovasc. Dev. Dis. 2023, 10, 213.
- Foote, C.A.; Soares, R.N.; Ramirez-Perez, F.I.; Ghiarone, T.; Aroor, A.; Manrique-Acevedo, C.; Padilla, J.; Martinez-Lemus, L. Endothelial Glycocalyx. Compr. Physiol. 2022, 12, 3781–3811.
- Pot, C.; Chen, A.Y.; Ha, J.N.; Schmid-Schönbein, G.W. Proteolytic Cleavage of the Red Blood Cell Glycocalyx in a Genetic Form of Hypertension. Cell. Mol. Bioeng. 2011, 4, 678–692.
- Salmon, A.H.; Ferguson, J.K.; Burford, J.L.; Gevorgyan, H.; Nakano, D.; Harper, S.J.; Harper, D.O. Bates, and J. Peti-Peterdi, Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J. Am. Soc. Nephrol. 2012, 23, 1339–1350.
- Tarbell, J.M.; Cancel, L.M. The glycocalyx and its significance in human medicine. J. Intern. Med. 2016, 280, 97–113.
- Esko, J.D.; Rostand, K.S.; Weinke, J.L. Tumor formation dependent on proteoglycan biosynthesis. Science 1988, 241, 1092–1096.
- Llaneza, A.; Vizoso, F.; Rodriguez, J.C.; Raigoso, P.; Garcia-Muniz, J.L.; Allende, M.T.; Garcia-Moran, M. Hyaluronic acid as prognostic marker in resectable colorectal cancer. Br. J. Surg. 2000, 87, 1690–1696.
- Adamia, S.; Maxwell, C.A.; Pilarski, L.M. Hyaluronan and hyaluronan synthases: Potential therapeutic targets in cancer. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005, 5, 3–14.
- Dogné, S.; Flamion, B. Endothelial Glycocalyx Impairment in Disease: Focus on Hyaluronan Shedding. Am. J. Pathol. 2020, 190, 768–780.
- Csóka, A.B.; Scherer, S.W.; Stern, R. Expression analysis of six paralogous human hyaluronidase genes clustered on chromosomes 3p21 and 7q31. Genomics 1999, 60, 356–361.
- Becker, B.F.; Jacob, M.; Leipert, S.; Salmon, A.H.; Chappell, D. Degradation of the endothelial glycocalyx in clinical settings: Searching for the sheddases. Br. J. Clin. Pharmacol. 2015, 80, 389–402.
- Johansson, P.I.; Stensballe, J.; Rasmussen, L.S.; Ostrowski, S.R. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann. Surg. 2011, 254, 194–200.
- Robich, M.; Ryzhov, S.; Kacer, D.; Palmeri, M.; Peterson, S.M.; Quinn, R.D.; Carter, D.; Sheppard, F.; Hayes, T.; Sawyer, D.B.; et al. Prolonged Cardiopulmonary Bypass is Associated with Endothelial Glycocalyx Degradation. J. Surg. Res. 2020, 251, 287–295.
- Gondelaud, F.; Ricard-Blum, S. Structures and interactions of syndecans. FEBS J. 2019, 286, 2994–3007.
- Couchman, J.R.; Gopal, S.; Lim, H.C.; Nørgaard, S.; Multhaupt, H.A. Fell-Muir Lecture: Syndecans: From peripheral coreceptors to mainstream regulators of cell behaviour. Int. J. Exp. Pathol. 2015, 96, 1–10.
- Deepa, S.S.; Yamada, S.; Zako, M.; Goldberger, O.; Sugahara, K. Chondroitin sulfate chains on syndecan-1 and syndecan-4 from normal murine mammary gland epithelial cells are structurally and functionally distinct and cooperate with heparan sulfate chains to bind growth factors. A novel function to control binding of midkine, pleiotrophin, and basic fibroblast growth factor. J. Biol. Chem. 2004, 279, 37368–37376.
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55.
- Bertrand, J.; Bollmann, M. Soluble syndecans: Biomarkers for diseases and therapeutic options. Br. J. Pharmacol. 2019, 176, 67–81.
- Pruessmeyer, J.; Martin, C.; Hess, F.M.; Schwarz, N.; Schmidt, S.; Kogel, T.; Hoettecke, N.; Schmidt, B.; Sechi, A.; Uhlig, S.; et al. A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J. Biol. Chem. 2010, 285, 555–564.
- Itoh, Y. Modulation of Microenvironment Signals by Proteolytic Shedding of Cell Surface Extracellular Matrix Receptors. Front. Cell Dev. Biol. 2021, 9, 736735.
- Filmus, J.; Capurro, M.; Rast, J. Glypicans. Genome Biol. 2008, 9, 224.
- Filmus, J. The function of glypicans in the mammalian embryo. Am. J. Physiol. Cell Physiol. 2022, 322, C694–C698.
- Filmus, J.; Capurro, M. The role of glypicans in Hedgehog signaling. Matrix Biol. 2014, 35, 248–252.
- Su, G.; Meyer, K.; Nandini, C.D.; Qiao, D.; Salamat, S.; Friedl, A. Glypican-1 is frequently overexpressed in human gliomas and enhances FGF-2 signaling in glioma cells. Am. J. Pathol. 2006, 168, 2014–2026.
- Traister, A.; Shi, W.; Filmus, J. Mammalian Notum induces the release of glypicans and other GPI-anchored proteins from the cell surface. Biochem. J. 2008, 410, 503–511.
- Zeng, Y. Endothelial glycocalyx as a critical signalling platform integrating the extracellular haemodynamic forces and chemical signalling. J. Cell. Mol. Med. 2017, 21, 1457–1462.
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525.
- Itano, N.; Sawai, T.; Yoshida, M.; Lenas, P.; Yamada, Y.; Imagawa, M.; Shinomura, T.; Hamaguchi, M.; Yoshida, Y.; Ohnuki, Y.; et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 1999, 274, 25085–25092.
- Rilla, K.; Oikari, S.; Jokela, T.A.; Hyttinen, J.M.; Kärnä, R.; Tammi, R.H.; Tammi, M.I. Hyaluronan synthase 1 (HAS1) requires higher cellular UDP-GlcNAc concentration than HAS2 and HAS3. J. Biol. Chem. 2013, 288, 5973–5983.
- Harada, H.; Takahashi, M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J. Biol. Chem. 2007, 282, 5597–5607.
- Kaul, A.; Short, W.D.; Wang, X.; Keswani, S.G. Hyaluronidases in Human Diseases. Int. J. Mol. Sci. 2021, 22, 3204.
- Patterson, E.K.; Cepinskas, G.; Fraser, D.D. Endothelial Glycocalyx Degradation in Critical Illness and Injury. Front. Med. 2022, 9, 898592.
- Anderegg, U.; Eichenberg, T.; Parthaune, T.; Haiduk, C.; Saalbach, A.; Milkova, L.; Ludwig, A.; Grosche, J.; Averbeck, M.; Gebhardt, C.; et al. ADAM10 is the constitutive functional sheddase of CD44 in human melanoma cells. J. Investig. Dermatol. 2009, 129, 1471–1482.
- Okamoto, I.; Kawano, Y.; Tsuiki, H.; Sasaki, J.I.; Nakao, M.; Matsumoto, M.; Suga, M.; Ando, M.; Nakajima, M.; Saya, H. CD44 cleavage induced by a membrane-associated metalloprotease plays a critical role in tumor cell migration. Oncogene 1999, 18, 1435–1446.
- Pardue, E.L.; Ibrahim, S.; Ramamurthi, A. Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering. Organogenesis 2008, 4, 203–214.
- Ding, H.Y.; Xie, Y.N.; Dong, Q.; Kimata, K.; Nishida, Y.; Ishiguro, N.; Zhuo, L.S. Roles of hyaluronan in cardiovascular and nervous system disorders. J. Zhejiang Univ. Sci. B 2019, 20, 428–436.
- West, D.C.; Kumar, S. Hyaluronan and angiogenesis. Ciba Found. Symp. 1989, 143, 187–201, discussion 81–85.
- Slevin, M.; Krupinski, J.; Gaffney, J.; Matou, S.; West, D.; Delisser, H.; Savani, R.C.; Kumar, S. Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007, 26, 58–68.
- Berdiaki, A.; Neagu, M.; Spyridaki, I.; Kuskov, A.; Perez, S.; Nikitovic, D. Hyaluronan and Reactive Oxygen Species Signaling-Novel Cues from the Matrix? Antioxidants 2023, 12, 824.
- Day, A.J.; de la Motte, C.A. Hyaluronan cross-linking: A protective mechanism in inflammation? Trends Immunol. 2005, 26, 637–643.
- Band, P.A.; Heeter, J.; Wisniewski, H.G.; Liublinska, V.; Pattanayak, C.W.; Karia, R.J.; Stabler, T.; Balazs, E.A.; Kraus, V.B. Hyaluronan molecular weight distribution is associated with the risk of knee osteoarthritis progression. Osteoarthr. Cartil. 2015, 23, 70–76.
- Heldin, P.; Kolliopoulos, C.; Lin, C.Y.; Heldin, C.H. Involvement of hyaluronan and CD44 in cancer and viral infections. Cell. Signal. 2020, 65, 109427.
- Karousou, E.; Misra, S.; Ghatak, S.; Dobra, K.; Götte, M.; Vigetti, D.; Passi, A.; Karamanos, N.K.; Skandalis, S.S. Roles and targeting of the HAS/hyaluronan/CD44 molecular system in cancer. Matrix Biol. 2017, 59, 3–22.
- Weinbaum, S.; Cancel, L.M.; Fu, B.M.; Tarbell, J.M. The Glycocalyx and Its Role in Vascular Physiology and Vascular Related Diseases. Cardiovasc. Eng. Technol. 2021, 12, 37–71.
- Ackerman, G. Serum Sodium. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Oxford, UK, 1990.
- Hyndman, K.A.; Mironova, E.V.; Giani, J.F.; Dugas, C.; Collins, J.; McDonough, A.A.; Stockand, J.D.; Pollock, J.S. Collecting Duct Nitric Oxide Synthase 1ß Activation Maintains Sodium Homeostasis During High Sodium Intake Through Suppression of Aldosterone and Renal Angiotensin II Pathways. J. Am. Heart Assoc. 2017, 6, e006896.
- Oberleithner, H.; Wilhelmi, M. Vascular glycocalyx sodium store—Determinant of salt sensitivity? Blood Purif. 2015, 39, 7–10.
- Korte, S.; Wiesinger, A.; Straeter, A.S.; Peters, W.; Oberleithner, H.; Kusche-Vihrog, K. Firewall function of the endothelial glycocalyx in the regulation of sodium homeostasis. Pflug. Arch. 2012, 463, 269–278.
- Nijst, P.; Verbrugge, F.H.; Grieten, L.; Dupont, M.; Steels, P.; Tang, W.H.W.; Mullens, W. The pathophysiological role of interstitial sodium in heart failure. J. Am. Coll. Cardiol. 2015, 65, 378–388.
- Titze, J.; Machnik, A. Sodium sensing in the interstitium and relationship to hypertension. Curr. Opin. Nephrol. Hypertens. 2010, 19, 385–392.
- Kusche-Vihrog, K.; Oberleithner, H. An emerging concept of vascular salt sensitivity. F1000 Biol. Rep. 2012, 4, 20.
- Oberleithner, H.; Peters, W.; Kusche-Vihrog, K.; Korte, S.; Schillers, H.; Kliche, K.; Oberleithner, K. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflug. Arch. 2011, 462, 519–528.
- Sulyok, E.; Farkas, B.; Nagy, B.; Várnagy, Á.; Kovács, K.; Bódis, J. Tissue Sodium Accumulation: Pathophysiology and Clinical Implications. Antioxidants 2022, 11, 750.
- Li, X.; Alu, A.; Wei, Y.; Wei, X.; Luo, M. The modulatory effect of high salt on immune cells and related diseases. Cell Prolif. 2022, 55, e13250.
- Kirabo, A. A new paradigm of sodium regulation in inflammation and hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R706–R710.
- Oberleithner, H.; Wälte, M.; Kusche-Vihrog, K. Sodium renders endothelial cells sticky for red blood cells. Front. Physiol. 2015, 6, 188.
- Mitra, R.; O’Neil, G.L.; Harding, I.C.; Cheng, M.J.; Mensah, S.A.; Ebong, E.E. Glycocalyx in Atherosclerosis-Relevant Endothelium Function and as a Therapeutic Target. Curr. Atheroscler. Rep. 2017, 19, 63.
- Mutchler, S.M.; Kleyman, T.R. New insights regarding epithelial Na+ channel regulation and its role in the kidney, immune system and vasculature. Curr. Opin. Nephrol. Hypertens. 2019, 28, 113–119.
- Ertuglu, L.A.; Kirabo, A. Dendritic Cell Epithelial Sodium Channel in Inflammation, Salt-Sensitive Hypertension, and Kidney Damage. Kidney360 2022, 3, 1620–1629.
- Barbaro, N.R.; Foss, J.D.; Kryshtal, D.O.; Tsyba, N.; Kumaresan, S.; Xiao, L.; Mernaugh, R.L.; Itani, H.A.; Loperena, R.; Chen, W.; et al. Dendritic Cell Amiloride-Sensitive Channels Mediate Sodium-Induced Inflammation and Hypertension. Cell Rep. 2017, 21, 1009–1020.
- Pitzer, A.; Elijovich, F.; Laffer, C.L.; Ertuglu, L.A.; Sahinoz, M.; Saleem, M.; Krishnan, J.; Dola, T.; Aden, L.A.; Sheng, Q.; et al. DC ENaC-Dependent Inflammasome Activation Contributes to Salt-Sensitive Hypertension. Circ. Res. 2022, 131, 328–344.
- Datla, S.R.; Griendling, K.K. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension 2010, 56, 325–330.
- Yen, W.; Cai, B.; Yang, J.; Zhang, L.; Zeng, M.; Tarbell, J.M.; Fu, B.M. Endothelial surface glycocalyx can regulate flow-induced nitric oxide production in microvessels in vivo. PLoS ONE 2015, 10, e0117133.
- Florian, J.A.; Kosky, J.R.; Ainslie, K.; Pang, Z.; Dull, R.O.; Tarbell, J.M. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ. Res. 2003, 93, e136–e142.
- Bartosch, A.M.W.; Mathews, R.; Tarbell, J.M. Endothelial Glycocalyx-Mediated Nitric Oxide Production in Response to Selective AFM Pulling. Biophys. J. 2017, 113, 101–108.
- Bhat, S.; Marklund, M.; Henry, M.E.; Appel, L.J.; Croft, K.D.; Neal, B.; Wu, J.H.Y. A Systematic Review of the Sources of Dietary Salt Around the World. Adv. Nutr. 2020, 11, 677–686.
- Hao, Z.; Liang, L.; Pu, D.; Zhang, Y. Analysis of Sodium Content in 4082 Kinds of Commercial Foods in China. Nutrients 2022, 14, 2908.
- Ojo, A.E.; Jones, A.; Okoro, C.E.; Alfa, V.O.; Okoli, R.; Shedul, G.L.; Orji, I.A.; Osagie, S.; Chopra, A.; Van Horn, L.V.; et al. Sodium Content and Labelling of Packaged Foods and Beverages in Nigeria: A Cross-Sectional Study. Nutrients 2022, 15, 27.
- Hunter, S.D.; Kavouras, S.A.; Rahimi, M. Exploring heated exercise as a means of preventing the deleterious effects of high-sodium intake in Black women. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H833–H839.
- Wenner, M.M.; Welti, L.M.; Dow, C.A.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Aerobic exercise training reduces ET-1-mediated vasoconstriction and improves endothelium-dependent vasodilation in postmenopausal women. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H732–H738.
- Dow, C.A.; Stauffer, B.L.; Brunjes, D.L.; Greiner, J.J.; DeSouza, C.A. Regular aerobic exercise reduces endothelin-1-mediated vasoconstrictor tone in overweight and obese adults. Exp. Physiol. 2017, 102, 1133–1142.
- Walker, J.C.; Dando, R. Sodium Replacement with KCl and MSG: Attitudes, Perception and Acceptance in Reduced Salt Soups. Foods 2023, 12, 2063.
- Lepedda, A.J.; Nieddu, G.; Piperigkou, Z.; Kyriakopoulou, K.; Karamanos, N.; Formato, M. Circulating Heparan Sulfate Proteoglycans as Biomarkers in Health and Disease. Semin. Thromb. Hemost. 2021, 47, 295–307.
- Huang, X.; Hu, H.; Sun, T.; Zhu, W.; Tian, H.; Hao, D.; Wang, T.; Wang, X. Plasma Endothelial Glycocalyx Components as a Potential Biomarker for Predicting the Development of Disseminated Intravascular Coagulation in Patients with Sepsis. J. Intensive Care Med. 2021, 36, 1286–1295.
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 16.
- Mony, V.K.; Benjamin, S.; O’Rourke, E.J. A lysosome-centered view of nutrient homeostasis. Autophagy 2016, 12, 619–631.
