Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Pancreatic cancer is challenging, with a poor progression and limited treatment options. Its tumorigenesis or metastasis involves, pathways, including phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), RAS, janus kinase (JAK)/signal transducer, and activator of transcription (STAT), NF-κB, Hippo/yes-kinase-associated protein (hippo/YAP), and Wingless/int1 (WNT). These pathways are associated with numerous cellular processes linked to pancreatic cancer, such as apoptosis, angiogenesis, differentiation, immunological regulations, metabolism, migration, and cell proliferation.
- molecular mechanism
- cancer
1. Introduction
Pancreatic cancer is a fatal disease that affects the pancreas, a large gland in the abdomen. American Cancer Society suggested that pancreatic cancer is the third highly common type of cancer in humans. Initial recognition is critical so that patients receive the extremely efficient treatment possible and have the greatest chance of survival [1,2,3]. Primarily, many patients are detected at an advanced stage. In the United States, this year approximately 53,090 people will be diagnosed with pancreatic cancer, and more than 41,170 people died from the disease [4,5]. Therefore, we must continue identifying new and improved ways to diagnose the disease earlier to save lives. Pancreatic cancer is highly destructive and has a poor prognosis with only a 9% of survival rate within five-years. Unluckily, the symptoms of pancreatic cancer are often vague and easy to overlook, so many people do not know that they have it until it is too late [6,7]. Its primary symptoms include jaundice, loss of appetite/weight, and right abdomen pain [4,8]. The pancreas is also challenging to reach and diagnose, so traditional imaging methods often cannot detect cancer early enough. However, early diagnosis and treatment are critical to improving survival. Today’s early detection methods are better than ever, but there are still significant limitations to available diagnostic tools. Methods of detecting pancreatic tumors include x-rays, ultrasounds, MRI.s, and CT scans [4]. These tests can effectively identify the tumor’s location and the extent of the disease. It is difficult to diagnose early, but specific blood tests can detect it if certain risk factors exist. Specific biomarkers can also be applied to detect cancer cells [9,10]. Pancreatic cancer is diagnosed with endoscopy, a minimally invasive procedure that uses a tube with a tiny camera attached to examine the inner lining of the digestive tract. During the process, removing a tissue sample from the pancreas to test for cancer cells can be validated [11,12]. Further, staging determines how far your cancer has spread and whether it has spread beyond the pancreas and risk assessment to effective diagnostic management [13].
The use of drugs destroys cancerous cells while sparing the surrounding healthy cells, allowing them to be combined with other cancer treatments to maximize their effectiveness [14,15]. Existing treatment possibilities for patients with advanced pancreatic cancer include chemotherapy, immunotherapy, targeted therapies, and surgery [4,16,17]. Chemotherapy involves using powerful drugs that kill cancer cells by attacking their DNA and disrupting their growth and division [18]. Gemcitabine is the first drug approved to treat pancreatic cancer [19]. It stops the development of new cancer cells and accelerates the immune system to kill them. The current standard treatment of gemcitabine is an intravenous injection twice a day for several days. After that, the treatment is repeated every three weeks until the patient no longer responds to the drug or until their condition worsens [19,20]. Unfortunately, few patients with advanced-stage cancer will respond to these treatments. New targeted therapies continue to be consideration-based in genomics, genetics, and molecular therapies, along with integrative concepts for improved diagnostics and treatments of pancreatic cancer [21,22,23].
Commonly known pancreatic tumors are pancreatic ductal adenocarcinoma (PDAC), accounting of nearly 90% of pancreatic-based cancers [24,25]. In some cases, patients may also develop secondary tumors in the liver or lungs due to the disease spreading throughout the body [26]. In general, treatment options for pancreatic cancer are usually limited and ineffective. Many patients with this disease do not respond to traditional treatments and are only given a few months to live [4,27]. However, recent developments in cancer treatment have led to many new therapies that may extend the lives of patients diagnosed with pancreatic cancer [28,29]. Furthermore, global perspectives have been significantly altered in the current pandemic toward therapeutic strategies for sustainable approaches to maintain good health and the economy [30,31].
2. Pancreatic Cancer Molecular Manifestation and Pathways Regulation
Pancreatic cancer is challenging, with a poor progression and limited treatment options [38]. Its tumorigenesis or metastasis involves, pathways, including phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), RAS, janus kinase (JAK)/signal transducer, and activator of transcription (STAT), NF-κB, Hippo/yes-kinase-associated protein (hippo/YAP), and Wingless/int1 (WNT). These pathways are associated with numerous cellular processes linked to pancreatic cancer, such as apoptosis, angiogenesis, differentiation, immunological regulations, metabolism, migration, and cell proliferation. In addition, histone modification is a vital feature in pancreatic cancer for epithelial-to-mesenchyme transition [4,39]. The regulation and consideration of these pathways for pancreatic cancer can be helpful in developing novel targets and therapeutics.
A key feature of pancreatic cancer includes the immunosuppressive tumor microenvironment [40,41]. Several molecular and cellular factors have been identified as critical players in the induction and maintenance of immunosuppression within the pancreatic stroma. The pancreatic stroma is composed of an extracellular matrix, immune cells, and fibroblasts that surround the tumor cells, forming a barrier that impedes cancer drug effect and immune cell infiltration [42] (Figure 1). Macrophages, which are a critical type of innate immune cells, play a significant role in immunomodulation in pancreatic cancer via a secreting range of cytokines [43]. Cytokines play a crucial role in tumor growth and immune cell evasion by promoting cancer cell invasion, proliferation, and immunosuppression [44,45]. Inflammatory cytokines such as IL-1β, IL-6, IL-8, and IL-10, have been demonstrated to activate tumor-associated macrophages (TAMs). The cytokines and chemokines attract immune cells, i.e., regulatory T cells (Treg cells), TAMs, and neutrophils, which impede CD8+ cytotoxic T cells function. Regulatory T cells (Tregs) have been associated with advancing pancreatic cancer by curbing cytotoxic T cells [46]. Pancreatic cancer cells coordinate immune evasion by synchronizing the secretion of cytokines in a highly coordinated way through TP53-dependent or KRAS-dependent pathways [47]. Tregs are vital in preserving immunologic self-tolerance and regulating suppression in pancreatic tumor growth.
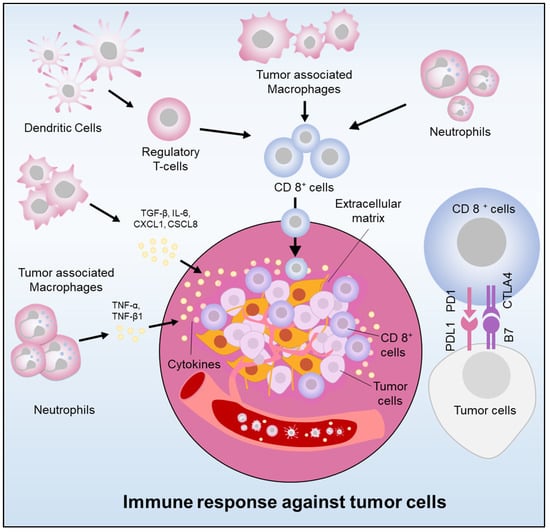
Figure 1. Immunoregulation in pancreatic cancer (Cancer cells elude the immune system by releasing cytokines and chemokines, recruiting immunosuppressive cells, and expressing PDL-1. Immune cell infiltration also stimulates tumor growth).
Recent studies have emphasized the vital importance of the cancer cell microenvironment in advancing cancer progression [48]. Moreover, the immune cells within the cancer cell microenvironment, including B cells, natural killer cells, T cells, and myeloid-derived suppressor cells are dysregulated and fail to mount an effective antitumor response [49]. The immunosuppressive cancer micro-environment shows a decisive role in the advancement of therapeutic resistance, which explains why current treatments are ineffective against pancreatic cancer. The progression of pancreatic cancer is modulated by various signaling pathways, including NF-κB, JAK/STAT, PI3K/AKT, Hippo/YAP, RAS, and WNT pathways (Figure 2). These pathways impact cellular functions such as apoptosis, differentiation, immunological regulation, metabolism, migration, angiogenesis, and cell proliferation [50,51,52,53]. Exploring and managing these signaling pathways has the potential to uncover novel targets and therapies for pancreatic cancer. RAS is a crucial driver of effector pathways, and its oncogenic activation is frequently found in pancreatic cancer, particularly in the KRAS isoform [47]. This activation can lead to cell proliferation, transformation, metastasis, and pro-inflammatory signaling activation. The PI3K/AKT pathway plays a significant role in pancreatic cancer regulation, with great potential for therapeutic targeting [54]. The PI3K/AKT pathway is commonly triggered in pancreatic cancer, with abnormal AKT overexpression associated with poor prognosis. PI3K signaling controls pancreatic cell plasticity and is activated early in tumor evolution [55]. This pathway is also activated by insulin-like growth factors and abnormal expression of various noncoding RNAs [56]. Several PI3K/AKT inhibitors are being investigated for their potential therapeutic effects in pancreatic cancer patients.

Figure 2. Molecular pathways of pancreatic cancer.
NF-κB is a crucial transcription-factor that shows a significant role in inflammation, is frequently stimulated in pancreatic cancer, and promotes cancer development, metastasis, and drug-resistance [57]. KRAS and other oncogenic mutations can also activate NF-κB. High quantities of chemokines are seen in pancreatic cancer, forming a positive feedback loop that enhances NF-κB signaling. Several ncRNAs can regulate the NF-κB pathway [58]. NF-κB is involved in antitumor immunity, and inhibitors of the pathway show promise as a therapeutic option. JAK/STAT pathways are engaged in a range of human cancers, together with pancreatic cancer, and increased JAK2 signaling indicates an inadequate prognosis of the disease [58,59]. The signaling pathway is implicated in inflammation in pancreatic cancer. In addition, interferons can increase PD-L1 expression via directly/indirectly influencing JAK-STAT signaling. Sustained JAK-STAT initiation can advance chronic inflammation and may impede CTL activation. JAK-STAT pathways have been associated with cancer developmental progress. YAP-TAZ are the major contributing factors of the Hippo pathway in pancreatic cancer [50]. Studies show that YAP is extremely articulated in patients and linked with an inadequate diagnosis of the disease. YAP is needed for cancer progression and is a crucial player in KRAS mutant mice. YAP can lead to disease relapse in the deficiency of KRAS and plays a vital driver of squamous pancreatic cancer. YAP-TAZ are transcriptional co-activators that can drive the gene expression engaged in proliferation and cell survival, impacting the hostile behavior of pancreatic cancerous cells. YAP also regulates the immunosuppressive microenvironment by modulating the behavior of PSCs and influencing the enlistment of TAMs and MDSCs. The WNT pathway controls somatic stem cells and is involved in pancreatic carcinogenesis and tumor progression through canonical and noncanonical pathways [60,61]. KRAS activation promotes pancreatic cancer cell movement and infiltration through the WNT pathway, and increased WNT/β-catenin signaling enhances the stem cell-like phenotypes. Both canonical and noncanonical WNT ligands have been linked to pancreatic cancer progression [60].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11061611
This entry is offline, you can click here to edit this entry!
