Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Many disorders of the anterior region of the eye may be efficiently treated via topical administration; however, it is more challenging to target conventional therapeutic doses to the posterior of the eye in this manner. Thus, various nanocarriers have been created and investigated for the transport of drugs and genes to the anterior or the posterior portions of the eyes. Liposomes, nanoparticles, micelles, inserts, implants, hydrogel, and emulsions are some of the most frequently utilized drug delivery systems.
- implants
- ocular delivery
1. Introduction
Many disorders of the anterior region of the eye may be efficiently treated via topical administration; however, it is more challenging to target conventional therapeutic doses to the posterior of the eye in this manner. Thus, various nanocarriers have been created and investigated for the transport of drugs and genes to the anterior or the posterior portions of the eyes. The most popular nano-drug delivery systems are depicted in Figure 1, and these can be utilized to increase the activity and bioavailability, and/or lessen the toxicity of the active pharmaceutical ingredients used. Liposomes, nanoparticles, micelles, inserts, implants, hydrogel, and emulsions are some of the most frequently utilized drug delivery systems.
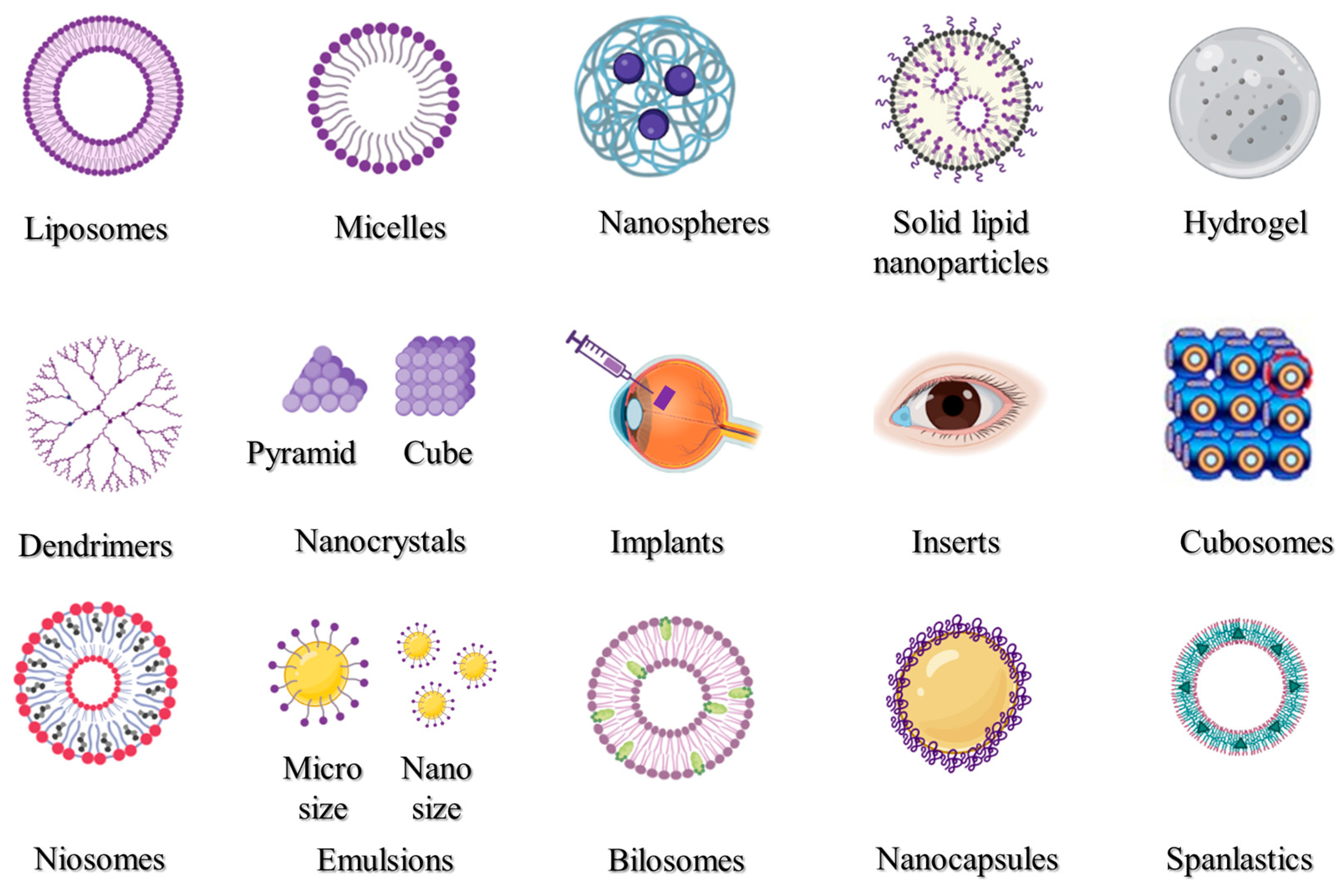
Figure 1. Nanocarrier systems investigated for ophthalmic uses.
2. Liposomes
Liposomes (Figure 1) are closed vesicles made of a phospholipid bilayer that can contain both drugs that are soluble in fat [1] and those that are soluble in water [2]. Due to their biodegradability, biocompatibility, and capacity to serve as drug carriers, liposomes have been thoroughly investigated for topical ocular administration (Table 1). Liposomes are particularly useful for large molecular weight and inadequately water-soluble drugs because they promote drug permeation through ocular tissues by virtue of their superior spreading ability and rheological properties that enable prolonging the drug availability on the surface of the eye [3][4]. Liposomes’ amphiphilic lipids form a tear compound-interacting sublayer when they make contact with tear lipid components. Polar heads and tails face the polar and non-polar tear lipid components, respectively, and help distribute the medication throughout the ocular surface [5]. Extensive research has examined the merits of liposomes for ocular use, minimizing potential drug toxicity and improving their absorption and bioavailability compared to an unencapsulated drug. These medications include vancomycin, tobramycin, ganciclovir, fluconazole, brinzolamide, triamcinolone acetonide, and cyclosporine A. Drug-loaded liposomal formulations injected intravitreally have a number of benefits. Some benefits include lengthening the half-life of drugs [6], safeguarding labile compounds [7], and prolonging the time that liposomes spend in the tissues of the eye [8].
3. Polymeric Micelles
Amphiphilic polymers can self-assemble into different structures, known as micelles (Figure 1). The formation of micelles within the nanometer range can efficiently improve the aqueous stability and enhance cell permeability. Prior research has demonstrated that the use of a nanomicelle formulation increased medication absorption in the eye [9][10]. Nanomicelle formulations are primarily used to improve the solubility of medications with low solubility and subsequently improve their bioavailability. Hesperidin, Sirolimus, Voriconazole, and Sunitinib are only a few of the medications that were made into polymeric nanomicelles with better solubility and therapeutic efficacy (Table 1).
4. Polymeric Nanoparticles
Polymeric nanoparticles (Figure 1) could be produced by the use of naturally occurring or synthetically produced polymers. Chitosan, hyaluronic acid, carboxymethylcellulose sodium, albumin, and sodium alginate are some examples of natural polymers, whereas poly(lactic-co-glycolic acid), poly(-caprolactone), and poly(ethylene glycol) are examples of synthetic polymers [11]. Some retinal drugs are currently not performing as expected due to the physical and chemical properties of the medications, as well as the distinctive anatomical structure of the eye. The bioavailability of these drugs was significantly increased [12], their toxicity was reduced [13], invasive procedures could be avoided [14], and pharmacokinetic modulation was achieved [15] via incorporation into polymeric nanoparticles (Table 1). These drugs include dexamethasone, cyclosporin, latanoprost, voriconazole, and ganciclovir. Hyaluronic acid, polyethylene glycol, and chitosan are examples of mucoadhesive polymers that may be employed to alter nanoparticles to lengthen their pericorneal residence duration [16]. Moreover, mucus penetrating nanoparticles, which possess low surface tension, low viscosity, and higher hydration water content, can enhance the penetration of therapeutic medicines through the cornea, increasing their bioavailability and resulting in better pharmacologic results. Consequently, mucus penetrating nanoparticles may significantly improve the treatment of posterior ocular problems, which include posterior uveitis, CMV, and retinal disorders [17].
5. Solid Lipid Nanoparticles
Lipids have been used to ameliorate the limited water solubility of several lipophilic drugs and adapt them as a drug delivery system [18]. Müller and Lucks initially developed solid lipid nanoparticles (SLNs, Figure 1) in 1996, which received the attention of scientists as a popular, stable, safe, and effective nanoscale drug delivery device. A surfactant layer that surrounds a solid lipid core in SLNs stabilizes and holds the medication [19]. Drug molecules can be found mostly in the center of particles or molecularly scattered throughout the matrix, depending on the drug solubility and the drug/lipid ratio [20]. SLNs are considered an efficient system intended for ocular drug delivery. SLNs can improve corneal drug absorptivity, enhance ocular tissue penetration and bioavailability, prolong residence time, and provide extended drug release properties [21]. SLNs were efficiently used to improve the delivery of bimatoprost [22], ofloxacin [23], and dorzolamide [24], as shown in Table 1.
6. Hydrogels
Hydrogels (Figure 1) are produced when polymeric solutions are crosslinked to form a network. The hydrogel complexation is formed on the basis of hydrophilic interactions between the polymer tail and water molecules [25]. Hydrogels are widely employed to provide ocularly applied or injectable dosage forms to a variety of eye regions. For ocular application, there are various hydrogel formulations that have FDA approval. A hydrogel sealant called ReSure® has been authorized for use in the non-operative treatment of clear corneal incisions. Hydrogels were also used to formulate and enhance the therapeutic activity of ocularly applied drugs, such as dexamethasone [26], bevacizumab [27], and timolol [28], as shown in Table 1.
7. Dendrimers
Dendrimers (Figure 1) are globular, negatively, positively, or neutrally branch-like nanostructured polymers. They derive their net charge from the functional groups, which are located at the ends of their branches [29]. These molecules consist of a fundamental unit called the “core”, which comprises the major component, and side chain units called “dendrons” [30]. Drugs may be conjugated to the ligands on the dendrimer surface or may be retained in the dendrimer core. Dendrimer manufacturing, generation, surface characteristics, and conjugation technique all have an impact on the drug-loading and drug-release kinetics of dendrimers [31]. As a result of their ability to selectively target inflammatory cells while causing no harm to healthy tissue, dendrimers have proven to be a viable drug delivery vehicle for the treatment of inflammatory eye conditions. The capacity to lower medication toxicity off-target is the key advantage of dendrimers’ targeting abilities [32]. Utilizing dendrimers effectively can increase the therapeutic effectiveness of various active pharmaceutical compounds (Table 1), including pilocarpine [33], tropicamide [33], dexamethasone [34], brimonidine, and timolol [35].
8. Nanocrystals
Nanocrystals (Figure 1) are crystals of therapeutic drugs with particle sizes as small as a few hundred nanometers, where pure drug crystals may occasionally be stabilized by the addition of surface active agents or polymeric solutions [36]. The benefits of nanocrystals over conventional nanocarriers, such as their high drug payload and comparative ease of manufacture, make them appealing candidates for the delivery of medications that are not readily water soluble [37][38]. The preparation of therapeutic drugs in the form of nanocrystals for ocular administration has various advantages. These advantages include better tolerability, increased ocular absorption, providing intermediated and prolonged release of drugs in the eye, and improved ocular permeation [39]. They also include improved ocular safety, increased formulation retention in cul-de-sac, and enhanced ocular permeation [40]. A number of medications used ocularly have been transformed into nanocrystals (Table 1) with enhanced properties, and these include dexamethasone [41], itraconazole [42], tedizolid [43], and brinzolamide [44]. Moreover, Novartis Pharmaceutical Corporation’s formulation of nepafenac nanocrystals received approval for commercial release (FDA, 2012) under the brand name Ilevro®.
9. Cubosomes
Cubosomes (Figure 1) are made up of two inner aqueous pathways that are separated into two arched interpenetrating lipid bilayers, which are structured in three dimensions resembling honeycombs [45]. These pathways can be occupied by a variety of bioactive molecules, including natural bioactives, chemical pharmaceuticals, peptides, polypeptides, and proteins [46]. Cubosomes are thought to be promising delivery systems because of their special characteristics, including thermodynamic stability, bioadhesion, the capacity to encapsulate different types of drugs, and their potential to control drug release [47]. Active medicines and macromolecules can successfully be applied topically to the posterior portion of the eye using cubosomes (Table 1). These drugs include beclomethasone [46], flurbiprofen [48], timolol [49], and brimonidine [50].
10. Niosomes
Niosomes, which are a type of vesicular system that includes a non-ionic surfactant, are closed bilayer structures produced once the nonionic surfactants self-assemble in an aqueous media to create nanocarriers (Figure 1). Researchers have begun using niosomal systems to treat severe inflammatory diseases and conditions, such various malignancies, because of their potential to boost the bioavailability and efficiency of the encapsulated therapeutics [51]. Niosomes are being investigated more and more for improving drug delivery to both segments of the eye, anterior and posterior, as well as promoting drug penetration and retention in ocular tissues. As a consequence, niosomes showed a considerable increase in the absorption and transcorneal permeability of topically applied drugs at the ocular surface (Table 1). These drugs include cyclopentolate [52], voriconazole [53], acetazolamide [54], gentamicin [55], brinzolamide [56], pilocarpine [57], and tacrolimus [58]. Additionally, niosomes, particularly charged vesicles, have been effectively used to transfer genes by subretinal or intravitreal injection to the retinal area [59].
11. Emulsions
An emulsion (Figure 1) is a uniform dispersion system that is formed upon mixing two or more immiscible liquids under certain circumstances [60]. Lipid-based emulsions have become a potential vehicle for ocular medication administration. The emulsions enhance ocular delivery using one of two major strategies, either by improving ocular permeability or by lengthening the period the formulation is retained on the ocular surface [61]. Both hydrophilic and lipophilic drug types may be loaded into emulsions [62][63]. Emulsions have been successfully used to create more effective formulations for several medications used intraocularly that have increased absorption and therapeutic effectiveness. These drugs include cyclosporine A [64], coumarin-6 [65], azithromycin, and disulfiram [66].
12. Bilosomes
One type of vesicular drug delivery system is the bilosome (Figure 1), which is made up of non-ionic amphiphilic compounds with integrated bile salt molecules. The negatively charged bile salts serve to maintain the bilosomal structure [67]. In comparison to niosomes and liposomes, these drug carriers are more stable and can effectively increase drug absorption through biological membranes [68]. Moreover, bilosomes can enhance the permeability of polysaccharides, proteins, and polypeptides, which are poorly transported through mucosal epithelial cells [69]. Previous research studies have assessed the effectiveness of bilosomes in the administration of ocular drugs (Table 1) and found that bilosomes are well tolerated by corneal tissues [70]. These drugs include terconazole [71], acetazolamide [70], ciprofloxacin [72], ciprofloxacin [72], agomelatine [73], and betaxolol [74].
13. Nanocapsules
Nanocapsules (Figure 1) are a subtype of nanoparticles that are comparable to vesicular systems, in which a medicine is contained in a hollow vessel with an inner liquid core encircled by a polymeric coating [75]. Nanocapsules are well-known to be retained in the cornea for a prolonged time and to enhance penetration throughout the deep ocular tissues [40]. Thus, the development of topically applied drug-loaded nanocapsules could reduce uncomfortable intravitreal injections and systemic delivery, which have serious side effects [40]. The therapeutic action of several medications was effectively potentiated via formulation in the form of nanocapsules (Table 1). These drugs include bevacizumab [76], prednisolone [77], tacrolimus [40], brinzolamide [44], and cyclosporine [78].
14. Spanlastics
Elastic niosomes, also known as spanlastics (Figure 1), are a subtype of vesicular drug delivery systems that are relatively new to the market. They resemble niosomes (non-ionic surfactant vesicles), except they contain an edge activator. They were first described as systems for ocular drug delivery [79], but since then, they have been used to deliver medications to a variety of bodily organs. The spanlastics’ bilayers become more elastic and deformable when an edge activator is present, which improves drug absorption across biological membranes. Spanlastics were efficiently used to payload hydrophilic, hydrophobic, and amphiphilic therapeutic pharmaceuticals for ocular use, especially the delivery to the posterior segment (Table 1). These drugs include ketoconazole [79], cyclosporine A [80], clotrimazole [81], and vanillic acid [82].
Table 1. Chronic eye conditions, available therapies, and drug delivery systems and their merits.
| Disease | Treatment | Drug | Delivery System Platform | Advantages of Delivery Systems In Vivo | Refs. | |
|---|---|---|---|---|---|---|
| Dry eye syndrome | Tear substitutes | Hypromellose | Solution | [83] | ||
| Methylcellulose and derivatives | Solution | [84] | ||||
| hyaluronic acid | Solution | [85] | ||||
| Aqueous secretagogues | Diquafosol sodium | Solution | [86] | |||
| Punctal plugs | Collagen and atelocollagen | In situ hydrogel | Prolonged activity | [87][88][89] | ||
| methacrylate-modified silk fibroin | In situ hydrogel | Prolonged activity | [90] | |||
| Mucin secretagogues | Rebamipide | Nanoparticles | Sustained release | [91] | ||
| Liposomes | Improved activity | [92] | ||||
| Micelles | Improved penetration | [93] | ||||
| Anti-inflammatory and immunomodulatory drugs | Cyclosporine | Micelles | Improved activity | [94] | ||
| Self-nanoemulsifying | Improved efficacy | [95] | ||||
| Liposomes | Improved activity | [96] | ||||
| Nanoparticles | Improved activity | [97] | ||||
| Nano-emulsion | Improved penetration | [98] | ||||
| Solid lipid nanoparticles | Controlled release | [99] | ||||
| In situ hydrogel | Improved activity | [94] | ||||
| Epigallocatechin gallate | Nanoparticles | Extended activity | [100] | |||
| In situ gels | Enhanced efficacy | [101] | ||||
| Lactoferrin | Nanoparticles | Enhanced efficacy | [102] | |||
| Nanocapsules | Controlled release | [103] | ||||
| Liposomes | Reduced irritation | [104] | ||||
| Nanostructured lipid carriers | Controlled release | [105] | ||||
| Vitamin A | Liposomes | Improved activity | [106] | |||
| Tacrolimus | Nanoparticles | Improved penetration | [107] | |||
| Progylcosomes | Improved activity | [108] | ||||
| Microcrystals | Improved efficacy | [109] | ||||
| Liposomes | Improved retention time | [110] | ||||
| Micelles | Prolonged activity | [111] | ||||
| Nanocapsules | Improved activity | [40] | ||||
| Corticosteroids | Dexamethasone | Dendrimer | Improved activity | [32] | ||
| Nano-wafer | Improved activity | [112] | ||||
| Nanostructured lipid carriers | Improved activity | [113] | ||||
| Nanoparticles | Improved penetration | [114] | ||||
| Micelles | Release modulation | [115] | ||||
| Nanosuspension | Prolonged activity | [116] | ||||
| Nano emulsion | Improved activity | [117] | ||||
| Nanosponges | Improved permeability | [118] | ||||
| Fluorometholone | Nanoparticles | Improved activity | [119] | |||
| Triamcinolone acetonide | Micelles | Release modulation | [120] | |||
| Nanoparticles | Improved activity | [121] | ||||
| Hydrocortisone | Nanosuspension | Prolonged activity | [116] | |||
| Micelles | Improved targeting | [122] | ||||
| Nanoparticles | Improved penetration | [122] | ||||
| Nanosuspension | Prolonged activity | [116] | ||||
| Prednisolone | Nanoparticles | Prolonged activity | [123] | |||
| Nano capsules | Reduced toxicity | [77] | ||||
| Lotep rednol etabonate |
Nanoparticles | Improved penetration | [124] | |||
| Non-steroidal anti-inflammatory drugs | Diclofenac sodium | Nanoparticles | Improved bioavailability | [125] | ||
| Nanosuspension | Prolonged activity | [126] | ||||
| Pranoprofen | Nanosuspension | Improved activity | [127] | |||
| Nanoparticles | Improved activity | [127][128] | ||||
| Bromfenac sodium | Liposomes | Extended release | [129] | |||
| Nanoparticles | Improved permeation | [130] | ||||
| Cubosomes | Improved bioavailability | [131] | ||||
| Ketorolac | Nanoparticles | Improved delivery | [132] | |||
| lymphocyte function-associated antigen-1 antagonists | Lifitegrast | Solution | [133][134] | |||
| Glaucoma | Prostaglandin analogues | Latanoprost | Nanoparticles | Controlled release | [135] | |
| PEGylated solid lipid | Improved permeability | [136] | ||||
| Micelles | Extended release | [137] | ||||
| Cubosomes | Sustained release | [138] | ||||
| Nanoparticles | Improved permeability | [139] | ||||
| Travoprost | Gold nanoparticles | Improved stability | [140] | |||
| Liposomes | Sustained release | [141] | ||||
| Spanlastics | Prolonged activity | [142] | ||||
| Nanoemulsion | Improved pharmacokinetics | [143] | ||||
| Implant | Controlled release | [144] | ||||
| Bimatoprost | Nanoparticles | Improved therapeutic activity | [22] | |||
| Gold nanoparticles | Controlled release | [145] | ||||
| Nanoparticle hydrogel | Controlled release | [146] | ||||
| Microemulsion | Improved permeability | [147] | ||||
| Graphene oxide-laden | Controlled release | [148] | ||||
| Implants | Sustained release | [149] | ||||
| Nanovesicular systems | Sustained release | [150] | ||||
| Inserts | Extended release | [151] | ||||
| Unoprostone | Transscleral device | Sustained release | [152] | |||
| Rho kinase inhibitors | Fasudil | Liposomes | Enhanced bioavailability | [153] | ||
| Microspheres | Sustained release | [154] | ||||
| Ripasudil | Solution | [155] | ||||
| Netarsudil | Solution | [156] | ||||
| β-adrenergic blockers | Timolol | Nanoparticles | Extended release | [157] | ||
| Micelles | Extended release | [137] | ||||
| Cubosomes | Improved bioavailability | [49] | ||||
| Nanogel | Sustained release | [158] | ||||
| Gelatinized core liposomes | Improved encapsulation | [159] | ||||
| Microemulsion | Improved bioavailability | [160] | ||||
| Levobunolol | Nanoparticles | Extended release | [161] | |||
| Microparticles | Sustained release | [162] | ||||
| Carteolol | Nanocapsules | Improved activity | [163] | |||
| Nanoparticles | Improved activity | [164] | ||||
| Chitosomes | Improved penetration | [165] | ||||
| Metipranolol | Nanocapsules | Reduced systemic side effects | [166] | |||
| Betaxolol | Liposomes | Extended activity | [167] | |||
| Nanoparticles | Controlled release | [168] | ||||
| Niosomes | Improved bioavailability | [169] | ||||
| Bilosomes | Improved transcorneal permeation | [74] | ||||
| α-adrenergic agonists | Brimonidine | Nanoparticles | Sustained release | [170] | ||
| Inserts | Controlled release | [171] | ||||
| Niosomes | Sustained release | [172] | ||||
| Microspheres | Sustained release | [173] | ||||
| Liposomes | Improved effectiveness | [174] | ||||
| Implant | Sustained release | [175] | ||||
| Gelatin-core liposomes | Improved drug loading | [176] | ||||
| Carbonic anhydrase inhibitors | Dorzolamide | Nanoparticles | Improved activity | [177] | ||
| Nanoemulsion | Enhanced ocular delivery | [178] | ||||
| Liposomes | Prolonged action | [179] | ||||
| Microparticles | Sustained release | [180] | ||||
| Niosomes | Improved activity | [181] | ||||
| Implant | Extended drug delivery | [182] | ||||
| Inserts | Improved activity | [183] | ||||
| Brinzolamide | Nanoparticles | Improved therapeutic activity | [184] | |||
| Nanocrystals | Improved penetration | [185] | ||||
| Liposomes | Sustained release | [186] | ||||
| Nanocapsules | Improved bioavailability | [44] | ||||
| Nanoemulsion | Improved therapeutic efficacy | [187] | ||||
| Nanofibers | Improved patient compliance | [188] | ||||
| Implant | Sustained release | [189] | ||||
| Acetazolamide | Cubosomes | Improved therapeutic efficacy | [190] | |||
| Spanlastics | Enhanced ocular delivery | [191] | ||||
| Transgelosomes | Enhanced ocular delivery | [192] | ||||
| Implants | Sustained release | [193] | ||||
| Niosomes | Improved permeability | [194] | ||||
| Bilosomes | Improved permeability | [70] | ||||
| Microsponges | Improved therapeutic efficacy | [195] | ||||
| Dendrimers | Sustained release | [196] | ||||
| Cholinergic agonists | Pilocarpine | Nanoparticles | Sustained release | [197] | ||
| Nanocapsules | Improved bioavailability | [198] | ||||
| Dendrimers | Prolonged residence time | [33] | ||||
| Uveitis | Corticosteroids | Fluocinolone acetonide | Implant (Retisert®) | Sustained release | [199] | |
| Nanoparticles | Improved bioavailability | [200] | ||||
| Difluprednate | Microneedles | Sustained release | [201] | |||
| Fluormetholone | Nanoparticles | Improved penetration | [202] | |||
| Nanocrystals | Improved sustained activity | [203] | ||||
| Triamcinolone acetonide | Nano lipid carriers | Improved penetration | [204] | |||
| Immunomodulator drugs | Adalimumab | Hydrogel | Improved permeability | [205] | ||
| Infliximab | Liposomes | Prolonged activity | [206] | |||
| Methotrexate | Implant | Sustained release | [207] | |||
| Sirolimus (Rapamycin) | Implant | Extended release | [208] | |||
| Micelles | Sustained release | [209] | ||||
| Exosomes | Improved therapeutic activity | [210] | ||||
| Liposomes | Improved therapeutic activity | [3] | ||||
| Endophthalmitis | Antimicrobials | Daptomycin | Nanoparticles | Noninvasive and improved activity | [211] | |
| Vancomycin | Nanostructured lipid carriers | Improved permeability and activity | [212] | |||
| Nanoparticles | Sustained release | [213] | ||||
| Thermoresponsive hydrogels | Controlled release | [214] | ||||
| Liposomes | Improved permeability | [215] | ||||
| Implant | Controlled release | [216] | ||||
| Niosomes | Improved permeability | [217] | ||||
| Ceftazidime | Nanoparticles | Improved activity and permeability | [218] | |||
| Antifungals | Amphotericin B | Liposomes | Improved activity-reduce toxicity | [219] | ||
| Voriconazole | Thermo-sensitive in situ gel | Sustained release | [220] | |||
| Nanoparticles | Improved permeability | [221] | ||||
| Microemulsion | Controlled release | [222] | ||||
| Elastosomes | Improved activity and reduced toxicity | [223] | ||||
| Micelles | Improved stability | [223] | ||||
| Liposomes | Improved permeability | [224] | ||||
| Antivirals | Cidofovir | Micelles | Prolonged activity | [225] | ||
| Liposomes | Prolonged activity | [226] | ||||
| Foscarnet | Liposomes | Improved activity and permeability | [227] | |||
| Ganciclovir | Nanoparticles | Sustained release | [228] | |||
| Glycerosomes | Sustained release | [229] | ||||
| Microemulsion | Improved permeability | [230] | ||||
| Vitrasert | Prolonged activity | [231] | ||||
| Minitablets | Sustained release | [232] | ||||
| Retinal diseases | Age-related macular degeneration | Anti-VEGF Agents | Ranibizumab | Nanoparticles | Improved activity | [233] |
| (Antibody fragment) | Microparticles | Improved intravitreal delivery | [234] | |||
| Liposomes | Increased encapsulation-release | [235] | ||||
| Quantum dots | Sustained release | [236] | ||||
| Implant | Sustained release | [237] | ||||
| Bevacizumab | Nanoparticles | Sustained delivery | [238] | |||
| (Monoclonal antibody) | Bi-layered capsule | Sustained delivery | [239] | |||
| Nanocapsules | Improved bioavailability | [240] | ||||
| Implant | Sustained release | [241] | ||||
| Microparticles | Sustained release | [242] | ||||
| Liposomes | Sustained release | [243] | ||||
| Aflibercept (VEGF-Trap) | Nanoparticles | Sustained drug release | [244] | |||
| Microspheres | Extended release | [245] | ||||
| Sunitinib | Nanoparticles | Superior prolonged activity | [246] | |||
| Micelles | Extended release | [247] | ||||
| Axitinib | Nanoparticles | Superior activity | [248] | |||
| Pegaptanib | PEGylated aptamer | Prolonged activity | [249] | |||
| Gene therapy | VEGF-siRNA | Liposomes | Improved activity-stability | [250] | ||
| Nanoball | Improved activity-targeting | [251] | ||||
| Nanoparticles | Improved therapeutic activity | [252] | ||||
| Integrin antagonists | C16Y peptide | Nanoparticles | Sustained release | [253] | ||
| Antioxidants | Serine-threonine-tyrosine peptide | Nanoparticles | Targeting | [254] | ||
| Resveratrol | Nanoparticles | Sustained release | [255] | |||
| Curcumin | Liposomes | Improved activity | [256] | |||
| Astragaloside | Nanocapsules | Improved activity | [257] | |||
| Diabetic retinopathy | Antiangiogenics | Anti-Flt1 peptide | Nanoparticles | Sustained release | [258] | |
| Micropump implant | On-demand targeting | [259] | ||||
| Fenofibrate | Nanoparticles | Controlled release | [260] | |||
| Pioglitazone | Nanoparticles | Controlled/improved activity | [261] | |||
| Apatinib | Nanoparticles | Improved activity | [262] | |||
| Silicate | Nanoparticles | Improved activity | [263] | |||
| Tacrolimus | Nanoparticles | Improved activity | [264] | |||
| Sorafenib tosylate | Nanoparticles | Improved activity | [265] | |||
| Octreotide | Nanoparticles | Improved activity-targeting | [266] | |||
| Anti-inflammatory and antioxidants | p-Coumaric acid | Nanoparticles | Improved activity | [267] | ||
| Connexin43 mimetic peptide | Nanoparticles | Targeting | [268] | |||
| Inulin D α-tocopherol succinate | Nanomicelles | Improved activity | [269] | |||
| Citicoline | Liposomes | Improved permeation | [270] | |||
| Melatonin | Nanoparticles | Controlled release and enhanced tolerability | [270] | |||
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15061746
References
- Mostafa, M.; Alaaeldin, E.; Aly, U.F.; Sarhan, H.A. Optimization and Characterization of Thymoquinone-Loaded Liposomes with Enhanced Topical Anti-inflammatory Activity. AAPS PharmSciTech 2018, 19, 3490–3500.
- Zheng, C.; Luo, W.; Liu, Y.; Chen, J.; Deng, H.; Zhou, Z.; Shen, J. Killing three birds with one stone: Multi-stage metabolic regulation mediated by clinically usable berberine liposome to overcome photodynamic immunotherapy resistance. Chem. Eng. J. 2023, 454, 140164.
- Suri, R.; Neupane, Y.R.; Mehra, N.; Jain, G.K.; Kohli, K. Sirolimus loaded polyol modified liposomes for the treatment of Posterior Segment Eye Diseases. Med. Hypotheses 2020, 136, 109518.
- Huang, X.; Li, M.; Bruni, R.; Messa, P.; Cellesi, F. The effect of thermosensitive liposomal formulations on loading and release of high molecular weight biomolecules. Int. J. Pharm. 2017, 524, 279–289.
- López-Cano, J.J.; González-Cela-Casamayor, M.A.; Andrés-Guerrero, V.; Herrero-Vanrell, R.; Molina-Martínez, I.T. Liposomes as vehicles for topical ophthalmic drug delivery and ocular surface protection. Expert Opin. Drug Deliv. 2021, 18, 819–847.
- Christensen, G.; Barut, L.; Urimi, D.; Schipper, N.; Paquet-Durand, F. Investigating Ex Vivo Animal Models to Test the Performance of Intravitreal Liposomal Drug Delivery Systems. Pharmaceutics 2021, 13, 1013.
- Salas-Ambrosio, P.J.; Bernad-Bernad, M.J.; Linares-Alba, M.A.; García-Santisteban, R.; Tonix-Aburto, L.A.; Ornelas-Lobato, G.J.; Gracia-Mora, I.; Rivera-Huerta, M.; Sánchez-Bartez, F.; Rico-Morales, H.; et al. Toxicity Evaluation of a Novel Rapamycin Liposomal Formulation After Subconjunctival and Intravitreal Injection. J. Ocul. Pharmacol. Ther. 2021, 37, 261–276.
- Blazaki, S.; Pachis, K.; Tzatzarakis, M.; Tsilimbaris, M.; Antimisiaris, S.G. Novel Liposome Aggregate Platform (LAP) system for sustained retention of drugs in the posterior ocular segment following intravitreal injection. Int. J. Pharm. 2020, 576, 118987.
- Terreni, E.; Chetoni, P.; Tampucci, S.; Burgalassi, S.; Al-kinani, A.A.; Alany, R.G.; Monti, D. Assembling Surfactants-Mucoadhesive Polymer Nanomicelles (ASMP-Nano) for Ocular Delivery of Cyclosporine-A. Pharmaceutics 2020, 12, 253.
- Binkhathlan, Z.; Alomrani, A.H.; Hoxha, O.; Ali, R.; Kalam, M.A.; Alshamsan, A. Development and Characterization of PEGylated Fatty Acid-Block-Poly(ε-caprolactone) Novel Block Copolymers and Their Self-Assembled Nanostructures for Ocular Delivery of Cyclosporine A. Polymers 2022, 14, 1635.
- Lynch, C.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Advances in Biodegradable Nano-Sized Polymer-Based Ocular Drug Delivery. Polymers 2019, 11, 1371.
- Suri, R.; Neupane, Y.R.; Mehra, N.; Nematullah, M.; Khan, F.; Alam, O.; Iqubal, A.; Jain, G.K.; Kohli, K. Sirolimus loaded chitosan functionalized poly (lactic-co-glycolic acid) (PLGA) nanoparticles for potential treatment of age-related macular degeneration. Int. J. Biol. Macromol. 2021, 191, 548–559.
- Romeo, A.; Musumeci, T.; Carbone, C.; Bonaccorso, A.; Corvo, S.; Lupo, G.; Anfuso, C.D.; Puglisi, G.; Pignatello, R. Ferulic Acid-Loaded Polymeric Nanoparticles for Potential Ocular Delivery. Pharmaceutics 2021, 13, 687.
- Bhatt, P.; Fnu, G.; Bhatia, D.; Shahid, A.; Sutariya, V. Nanodelivery of Resveratrol-Loaded PLGA Nanoparticles for Age-Related Macular Degeneration. AAPS PharmSciTech 2020, 21, 291.
- Mahaling, B.; Baruah, N.; Ahamad, N.; Maisha, N.; Lavik, E.; Katti, D.S. A non-invasive nanoparticle-based sustained dual-drug delivery system as an eyedrop for endophthalmitis. Int. J. Pharm. 2021, 606, 120900.
- Bu, H.-Z.; Gukasyan, H.J.; Goulet, L.; Lou, X.-J.; Xiang, C.; Koudriakova, T. Ocular Disposition, Pharmacokinetics, Efficacy and Safety of Nanoparticle-Formulated Ophthalmic Drugs. Curr. Drug Metab. 2007, 8, 91–107.
- Yan, R.; Xu, L.; Wang, Q.; Wu, Z.; Zhang, H.; Gan, L. Cyclosporine A Nanosuspensions for Ophthalmic Delivery: A Comparative Study between Cationic Nanoparticles and Drug-Core Mucus Penetrating Nanoparticles. Mol. Pharm. 2021, 18, 4290–4298.
- Shah, S.; Bhanderi, B.; Soniwala, M.; Chavda, J. Lutein-Loaded Solid Lipid Nanoparticles for Ocular Delivery: Statistical Optimization and Ex Vivo Evaluation. J. Pharm. Innov. 2022, 17, 584–598.
- Onugwu, A.L.; Attama, A.A.; Nnamani, P.O.; Onugwu, S.O.; Onuigbo, E.B.; Khutoryanskiy, V.V. Development and optimization of solid lipid nanoparticles coated with chitosan and poly(2-ethyl-2-oxazoline) for ocular drug delivery of ciprofloxacin. J. Drug Deliv. Sci. Technol. 2022, 74, 103527.
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303.
- Alhakamy, N.A.; Hosny, K.M.; Aldryhim, A.Y.; Rizg, W.Y.; Eshmawi, B.A.; Bukhary, H.A.; Murshid, S.S.A.; Khallaf, R.A. Development and optimization of ofloxacin as solid lipid nanoparticles for enhancement of its ocular activity. J. Drug Deliv. Sci. Technol. 2022, 72, 103373.
- Wadetwar, R.N.; Agrawal, A.R.; Kanojiya, P.S. In situ gel containing Bimatoprost solid lipid nanoparticles for ocular delivery: In-vitro and ex-vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 56, 101575.
- Eid, H.M.; Elkomy, M.H.; El Menshawe, S.F.; Salem, H.F. Development, Optimization, and In Vitro/In Vivo Characterization of Enhanced Lipid Nanoparticles for Ocular Delivery of Ofloxacin: The Influence of Pegylation and Chitosan Coating. AAPS PharmSciTech 2019, 20, 183.
- Shahab, M.S.; Rizwanullah, M.; Sarim Imam, S. Formulation, optimization and evaluation of vitamin E TPGS emulsified dorzolamide solid lipid nanoparticles. J. Drug Deliv. Sci. Technol. 2022, 68, 103062.
- Fathi, M.; Barar, J.; Aghanejad, A.; Omidi, Y. Hydrogels for ocular drug delivery and tissue engineering. BioImpacts BI 2015, 5, 159–164.
- Annala, A.; Ilochonwu, B.C.; Wilbie, D.; Sadeghi, A.; Hennink, W.E.; Vermonden, T. Self-Healing Thermosensitive Hydrogel for Sustained Release of Dexamethasone for Ocular Therapy. ACS Polym. Au 2023, 3, 118–131.
- Ilochonwu, B.C.; Mihajlovic, M.; Maas-Bakker, R.F.; Rousou, C.; Tang, M.; Chen, M.; Hennink, W.E.; Vermonden, T. Hyaluronic Acid-PEG-Based Diels–Alder In Situ Forming Hydrogels for Sustained Intraocular Delivery of Bevacizumab. Biomacromolecules 2022, 23, 2914–2929.
- Pakzad, Y.; Fathi, M.; Omidi, Y.; Mozafari, M.; Zamanian, A. Synthesis and characterization of timolol maleate-loaded quaternized chitosan-based thermosensitive hydrogel: A transparent topical ocular delivery system for the treatment of glaucoma. Int. J. Biol. Macromol. 2020, 159, 117–128.
- Xu, Q.; Kambhampati, S.P.; Kannan, R.M. Nanotechnology approaches for ocular drug delivery. Middle East Afr. J. Ophthalmol. 2013, 20, 26–37.
- Honda, M.; Asai, T.; Oku, N.; Araki, Y.; Tanaka, M.; Ebihara, N. Liposomes and nanotechnology in drug development: Focus on ocular targets. Int. J. Nanomed. 2013, 8, 495–504.
- Rodríguez Villanueva, J.; Navarro, M.G.; Rodríguez Villanueva, L. Dendrimers as a promising tool in ocular therapeutics: Latest advances and perspectives. Int. J. Pharm. 2016, 511, 359–366.
- Soiberman, U.; Kambhampati, S.P.; Wu, T.; Mishra, M.K.; Oh, Y.; Sharma, R.; Wang, J.; Al Towerki, A.E.; Yiu, S.; Stark, W.J.; et al. Subconjunctival injectable dendrimer-dexamethasone gel for the treatment of corneal inflammation. Biomaterials 2017, 125, 38–53.
- Vandamme, T.F.; Brobeck, L. Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J. Control. Release 2005, 102, 23–38.
- Yavuz, B.; Pehlivan, S.B.; Vural, İ.; Ünlü, N. In Vitro/In Vivo Evaluation of Dexamethasone—PAMAM Dendrimer Complexes for Retinal Drug Delivery. J. Pharm. Sci. 2015, 104, 3814–3823.
- Wang, J.; Li, B.; Huang, D.; Norat, P.; Grannonico, M.; Cooper, R.C.; Gui, Q.; Nam Chow, W.; Liu, X.; Yang, H. Nano-in-Nano dendrimer gel particles for efficient topical delivery of antiglaucoma drugs into the eye. Chem. Eng. J. 2021, 425, 130498.
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities. Nanomaterials 2020, 10, 847.
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Zhou, L.; Li, X.; Wang, F. Application of Drug Nanocrystal Technologies on Oral Drug Delivery of Poorly Soluble Drugs. Pharm. Res. 2013, 30, 307–324.
- McGuckin, M.B.; Wang, J.; Ghanma, R.; Qin, N.; Palma, S.D.; Donnelly, R.F.; Paredes, A.J. Nanocrystals as a master key to deliver hydrophobic drugs via multiple administration routes. J. Control. Release 2022, 345, 334–353.
- Sharma, O.P.; Patel, V.; Mehta, T. Nanocrystal for ocular drug delivery: Hope or hype. Drug Deliv. Transl. Res. 2016, 6, 399–413.
- Rebibo, L.; Tam, C.; Sun, Y.; Shoshani, E.; Badihi, A.; Nassar, T.; Benita, S. Topical tacrolimus nanocapsules eye drops for therapeutic effect enhancement in both anterior and posterior ocular inflammation models. J. Control. Release 2021, 333, 283–297.
- Romero, G.B.; Keck, C.M.; Müller, R.H.; Bou-Chacra, N.A. Development of cationic nanocrystals for ocular delivery. Eur. J. Pharm. Biopharm. 2016, 107, 215–222.
- Tetyczka, C.; Brisberger, K.; Reiser, M.; Zettl, M.; Jeitler, R.; Winter, C.; Kolb, D.; Leitinger, G.; Spoerk, M.; Roblegg, E. Itraconazole Nanocrystals on Hydrogel Contact Lenses via Inkjet Printing: Implications for Ophthalmic Drug Delivery. ACS Appl. Nano Mater. 2022, 5, 9435–9446.
- Kalam, M.A.; Iqbal, M.; Alshememry, A.; Alkholief, M.; Alshamsan, A. Fabrication and Characterization of Tedizolid Phosphate Nanocrystals for Topical Ocular Application: Improved Solubilization and In Vitro Drug Release. Pharmaceutics 2022, 14, 1328.
- Dubey, V.; Mohan, P.; Dangi, J.S.; Kesavan, K. Brinzolamide loaded chitosan-pectin mucoadhesive nanocapsules for management of glaucoma: Formulation, characterization and pharmacodynamic study. Int. J. Biol. Macromol. 2020, 152, 1224–1232.
- Tan, C.; Hosseini, S.F.; Jafari, S.M. Cubosomes and Hexosomes as Novel Nanocarriers for Bioactive Compounds. J. Agric. Food Chem. 2022, 70, 1423–1437.
- Gaballa, S.A.; El Garhy, O.H.; Moharram, H.; Abdelkader, H. Preparation and Evaluation of Cubosomes/Cubosomal Gels for Ocular Delivery of Beclomethasone Dipropionate for Management of Uveitis. Pharm. Res. 2020, 37, 198.
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable drug delivery potential. Drug Discov. Today 2016, 21, 789–801.
- Han, S.; Shen, J.-Q.; Gan, Y.; Geng, H.-M.; Zhang, X.-X.; Zhu, C.-L.; Gan, L. Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharmacol. Sin. 2010, 31, 990–998.
- Huang, J.; Peng, T.; Li, Y.; Zhan, Z.; Zeng, Y.; Huang, Y.; Pan, X.; Wu, C.-Y.; Wu, C. Ocular Cubosome Drug Delivery System for Timolol Maleate: Preparation, Characterization, Cytotoxicity, Ex Vivo, and In Vivo Evaluation. AAPS PharmSciTech 2017, 18, 2919–2926.
- Eldeeb, A.E.; Salah, S.; Ghorab, M. Formulation and evaluation of cubosomes drug delivery system for treatment of glaucoma: Ex-vivo permeation and in-vivo pharmacodynamic study. J. Drug Deliv. Sci. Technol. 2019, 52, 236–247.
- Yasamineh, S.; Yasamineh, P.; Ghafouri Kalajahi, H.; Gholizadeh, O.; Yekanipour, Z.; Afkhami, H.; Eslami, M.; Hossein Kheirkhah, A.; Taghizadeh, M.; Yazdani, Y.; et al. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int. J. Pharm. 2022, 624, 121878.
- Saettone, M.F.; Perini, G.; Carafa, M.; Santucci, E.; Alhaique, F. Nonionic surfactant vesicles as ophthalmic carriers for cyclopentolate: Preliminary evaluation. STP Pharma. Sci. 1996, 6, 94–98.
- Shukr, M.H. Novel in situ gelling ocular inserts for voriconazole-loaded niosomes: Design, in vitro characterisation and in vivo evaluation of the ocular irritation and drug pharmacokinetics. J. Microencapsul. 2016, 33, 71–79.
- Aggarwal, D.; Pal, D.; Mitra, A.K.; Kaur, I.P. Study of the extent of ocular absorption of acetazolamide from a developed niosomal formulation, by microdialysis sampling of aqueous humor. Int. J. Pharm. 2007, 338, 21–26.
- Abdelbary, G.; El-gendy, N. Niosome-Encapsulated Gentamicin for Ophthalmic Controlled Delivery. AAPS PharmSciTech 2008, 9, 740–747.
- Gupta, P.; Yadav, K.S. Formulation and evaluation of brinzolamide encapsulated niosomal in-situ gel for sustained reduction of IOP in rabbits. J. Drug Deliv. Sci. Technol. 2022, 67, 103004.
- Owodeha-Ashaka, K.; Ilomuanya, M.O.; Iyire, A. Evaluation of sonication on stability-indicating properties of optimized pilocarpine hydrochloride-loaded niosomes in ocular drug delivery. Prog. Biomater. 2021, 10, 207–220.
- Zeng, W.; Li, Q.; Wan, T.; Liu, C.; Pan, W.; Wu, Z.; Zhang, G.; Pan, J.; Qin, M.; Lin, Y.; et al. Hyaluronic acid-coated niosomes facilitate tacrolimus ocular delivery: Mucoadhesion, precorneal retention, aqueous humor pharmacokinetics, and transcorneal permeability. Colloids Surf. B Biointerfaces 2016, 141, 28–35.
- Puras, G.; Mashal, M.; Zárate, J.; Agirre, M.; Ojeda, E.; Grijalvo, S.; Eritja, R.; Diaz-Tahoces, A.; Martínez Navarrete, G.; Avilés-Trigueros, M.; et al. A novel cationic niosome formulation for gene delivery to the retina. J. Control. Release 2014, 174, 27–36.
- Peng, C.C.; Bengani, L.C.; Jung, H.J.; Leclerc, J.; Gupta, C.; Chauhan, A. Emulsions and microemulsions for ocular drug delivery. J. Drug Deliv. Sci. Technol. 2011, 21, 111–121.
- Tiwari, R.; Pandey, V.; Asati, S.; Soni, V.; Jain, D. Therapeutic challenges in ocular delivery of lipid based emulsion. Egypt. J. Basic Appl. Sci. 2018, 5, 121–129.
- Nair, R.; Chakrapani, M.; Kaza, R. Preparation and Evaluation of Vancomycin Microemulsion for Ocular Drug Delivery. Drug Deliv. Lett. 2012, 2, 26–34.
- Ameta, R.K.; Soni, K.; Bhattarai, A. Recent Advances in Improving the Bioavailability of Hydrophobic/Lipophilic Drugs and Their Delivery via Self-Emulsifying Formulations. Colloids Interfaces 2023, 7, 16.
- Li, Y.; Guan, Q.; Xu, J.; Zhang, H.; Liu, S.; Ding, Z.; Wang, Q.; Wang, Z.; Liu, M.; Zhao, Y. Comparative study of cyclosporine A liposomes and emulsions for ophthalmic drug delivery: Process optimization through response surface methodology (RSM) and biocompatibility evaluation. Colloids Surf. B Biointerfaces 2023, 225, 113267.
- Ying, L.; Tahara, K.; Takeuchi, H. Drug delivery to the ocular posterior segment using lipid emulsion via eye drop administration: Effect of emulsion formulations and surface modification. Int. J. Pharm. 2013, 453, 329–335.
- Liu, C.; Lan, Q.; He, W.; Nie, C.; Zhang, C.; Xu, T.; Jiang, T.; Wang, S. Octa-arginine modified lipid emulsions as a potential ocular delivery system for disulfiram: A study of the corneal permeation, transcorneal mechanism and anti-cataract effect. Colloids Surf. B Biointerfaces 2017, 160, 305–314.
- Ahad, A.; Raish, M.; Ahmad, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Eprosartan mesylate loaded bilosomes as potential nano-carriers against diabetic nephropathy in streptozotocin-induced diabetic rats. Eur. J. Pharm. Sci. 2018, 111, 409–417.
- Mondal, D.; Mandal, R.P.; De, S. Addressing the Superior Drug Delivery Performance of Bilosomes─A Microscopy and Fluorescence Study. ACS Appl. Bio. Mater. 2022, 5, 3896–3911.
- Csaba, N.; Garcia-Fuentes, M.; Alonso, M.J. The performance of nanocarriers for transmucosal drug delivery. Expert Opin. Drug Deliv. 2006, 3, 463–478.
- Mohsen, A.M.; Salama, A.; Kassem, A.A. Development of acetazolamide loaded bilosomes for improved ocular delivery: Preparation, characterization and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 59, 101910.
- Abdelbary, A.A.; Abd-Elsalam, W.H.; Al-mahallawi, A.M. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: In vitro characterization, ex vivo permeation and in vivo safety assessment. Int. J. Pharm. 2016, 513, 688–696.
- Alsaidan, O.A.; Zafar, A.; Yasir, M.; Alzarea, S.I.; Alqinyah, M.; Khalid, M. Development of Ciprofloxacin-Loaded Bilosomes In-Situ Gel for Ocular Delivery: Optimization, In-Vitro Characterization, Ex-Vivo Permeation, and Antimicrobial Study. Gels 2022, 8, 687.
- Nemr, A.A.; El-Mahrouk, G.M.; Badie, H.A. Hyaluronic acid-enriched bilosomes: An approach to enhance ocular delivery of agomelatine via D-optimal design: Formulation, in vitro characterization, and in vivo pharmacodynamic evaluation in rabbits. Drug Deliv. 2022, 29, 2343–2356.
- Sakr, M.G.; El-Zahaby, S.A.; Al-Mahallawi, A.M.; Ghorab, D.M. Fabrication of betaxolol hydrochloride-loaded highly permeable ocular bilosomes (HPOBs) to combat glaucoma: In vitro, ex vivo & in vivo characterizations. J. Drug Deliv. Sci. Technol. 2023, 82, 104363.
- Quintanar-Guerrero, D.; Allémann, E.; Fessi, H.; Doelker, E. Preparation Techniques and Mechanisms of Formation of Biodegradable Nanoparticles from Preformed Polymers. Drug Dev. Ind. Pharm. 1998, 24, 1113–1128.
- Formica, M.L.; Legeay, S.; Bejaud, J.; Montich, G.G.; Ullio Gamboa, G.V.; Benoit, J.-P.; Palma, S.D. Novel hybrid lipid nanocapsules loaded with a therapeutic monoclonal antibody–Bevacizumab–and Triamcinolone acetonide for combined therapy in neovascular ocular pathologies. Mater. Sci. Eng. C 2021, 119, 111398.
- Katzer, T.; Chaves, P.; Bernardi, A.; Pohlmann, A.; Guterres, S.S.; Ruver Beck, R.C. Prednisolone-loaded nanocapsules as ocular drug delivery system: Development, in vitro drug release and eye toxicity. J. Microencapsul. 2014, 31, 519–528.
- Eldesouky, L.M.; El-Moslemany, R.M.; Ramadan, A.A.; Morsi, M.H.; Khalafallah, N.M. Cyclosporine Lipid Nanocapsules as Thermoresponsive Gel for Dry Eye Management: Promising Corneal Mucoadhesion, Biodistribution and Preclinical Efficacy in Rabbits. Pharmaceutics 2021, 13, 360.
- Kakkar, S.; Kaur, I.P. Spanlastics—A novel nanovesicular carrier system for ocular delivery. Int. J. Pharm. 2011, 413, 202–210.
- Liu, Y.; Wang, Y.; Yang, J.; Zhang, H.; Gan, L. Cationized hyaluronic acid coated spanlastics for cyclosporine A ocular delivery: Prolonged ocular retention, enhanced corneal permeation and improved tear production. Int. J. Pharm. 2019, 565, 133–142.
- Abdelbari, M.A.; El-Mancy, S.S.; Elshafeey, A.H.; Abdelbary, A.A. Implementing Spanlastics for Improving the Ocular Delivery of Clotrimazole: In vitro Characterization, Ex vivo Permeability, Microbiological Assessment and In vivo Safety Study. Int. J. Nanomed. 2021, 16, 6249–6261.
- Ibrahim, S.S.; Abd-allah, H. Spanlastic nanovesicles for enhanced ocular delivery of vanillic acid: Design, in vitro characterization, and in vivo anti-inflammatory evaluation. Int. J. Pharm. 2022, 625, 122068.
- Tauber, J. Efficacy, tolerability and comfort of a 0.3% hypromellose gel ophthalmic lubricant in the treatment of patients with moderate to severe dry eye syndrome. Curr. Med. Res. Opin. 2007, 23, 2629–2636.
- Rajendraprasad, R.M.; Kwatra, G.; Batra, N. Carboxymethyl Cellulose versus Hydroxypropyl Methylcellulose Tear Substitutes for Dry Eye Due to Computer Vision Syndrome: Comparison of Efficacy and Safety. Int. J. Appl. Basic Med. Res. 2021, 11, 4–8.
- Hynnekleiv, L.; Magno, M.; Vernhardsdottir, R.R.; Moschowits, E.; Tønseth, K.A.; Dartt, D.A.; Vehof, J.; Utheim, T.P. Hyaluronic acid in the treatment of dry eye disease. Acta Ophthalmol. 2022, 100, 844–860.
- Sun, X.; Liu, L.; Liu, C. Topical diquafosol versus hyaluronic acid for the treatment of dry eye disease: A meta-analysis of randomized controlled trials. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 1–13.
- Modi, D.; Nirmal, J.; Warsi, M.H.; Bhatia, M.; Hasan, N.; Kesharwani, P.; Jain, G.K. Formulation and development of tacrolimus-gellan gum nanoformulation for treatment of dry eye disease. Colloids Surf. B Biointerfaces 2022, 211, 112255.
- Pearce, E.I.; Dorman, M.; Wilkinson, B.C.; Oliver, K.M. Effect of Blink Frequency on Tear Turnover Rate. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3726.
- Chen, M.; Yung Choi, S. Preliminary Outcomes of Temporary Collagen Punctal Plugs for Patients with Dry Eye and Glaucoma. Med. Hypothesis Discov. Innov. Ophthalmol. J. 2020, 9, 56–60.
- Dai, M.; Xu, K.; Xiao, D.; Zheng, Y.; Zheng, Q.; Shen, J.; Qian, Y.; Chen, W. In Situ Forming Hydrogel as a Tracer and Degradable Lacrimal Plug for Dry Eye Treatment. Adv. Healthc. Mater. 2022, 11, 2200678.
- Nagai, N.; Ishii, M.; Seiriki, R.; Ogata, F.; Otake, H.; Nakazawa, Y.; Okamoto, N.; Kanai, K.; Kawasaki, N. Novel Sustained-Release Drug Delivery System for Dry Eye Therapy by Rebamipide Nanoparticles. Pharmaceutics 2020, 12, 155.
- Qiao, H.; Xu, Z.; Sun, M.; Fu, S.; Zhao, F.; Wang, D.; He, Z.; Zhai, Y.; Sun, J. Rebamipide liposome as an effective ocular delivery system for the management of dry eye disease. J. Drug Deliv. Sci. Technol. 2022, 75, 103654.
- Li, Q.; Wu, X.; Xin, M. Strengthened rebamipide ocular nanoformulation to effectively treat corneal alkali burns in mice through the HMGB1 signaling pathway. Exp. Eye Res. 2021, 213, 108824.
- Terreni, E.; Zucchetti, E.; Tampucci, S.; Burgalassi, S.; Monti, D.; Chetoni, P. Combination of Nanomicellar Technology and In Situ Gelling Polymer as Ocular Drug Delivery System (ODDS) for Cyclosporine-A. Pharmaceutics 2021, 13, 192.
- Bang, S.P.; Yeon, C.Y.; Adhikari, N.; Neupane, S.; Kim, H.; Lee, D.C.; Son, M.J.; Lee, H.G.; Kim, J.-Y.; Jun, J.H. Cyclosporine A eyedrops with self-nanoemulsifying drug delivery systems have improved physicochemical properties and efficacy against dry eye disease in a murine dry eye model. PLoS ONE 2019, 14, e0224805.
- Wong, K.-Y.; Liu, Y.; Zhou, L.; Wong, M.-S.; Liu, J. Mucin-targeting-aptamer functionalized liposomes for delivery of cyclosporin A for dry eye diseases. J. Mater. Chem. B 2023, 11.
- Chhowala, I.; Patel, A.; Patel, R.; Bhavsar, V.; Dharamsi, A. Optimisation of PCL-HA laden biodegradable nanoparticles containing cyclosporine-A for the treatment of dry eye syndrome: In vitro-in vivo evaluation. Int. J. Nanoparticles 2021, 13, 106–120.
- Lallemand, F.; Daull, P.; Benita, S.; Buggage, R.; Garrigue, J.-S.J.J.O.D.D. Successfully improving ocular drug delivery using the cationic nanoemulsion, novasorb. J. Drug Deliv. 2012, 2012, 604204.
- Gökçe, E.H.; Sandri, G.; Eğrilmez, S.; Bonferoni, M.C.; Güneri, T.; Caramella, C. Cyclosporine A-Loaded Solid Lipid Nanoparticles: Ocular Tolerance and In Vivo Drug Release in Rabbit Eyes. Curr. Eye Res. 2009, 34, 996–1003.
- Huang, H.Y.; Wang, M.C.; Chen, Z.Y.; Chiu, W.Y.; Chen, K.H.; Lin, I.C.; Yang, W.V.; Wu, C.C.; Tseng, C.L. Gelatin-epigallocatechin gallate nanoparticles with hyaluronic acid decoration as eye drops can treat rabbit dry-eye syndrome effectively via inflammatory relief. Int. J. Nanomed. 2018, 13, 7251–7273.
- Luo, L.-J.; Lai, J.-Y. Epigallocatechin Gallate-Loaded Gelatin-g-Poly(N-Isopropylacrylamide) as a New Ophthalmic Pharmaceutical Formulation for Topical Use in the Treatment of Dry Eye Syndrome. Sci. Rep. 2017, 7, 9380.
- López-Machado, A.; Díaz, N.; Cano, A.; Espina, M.; Badía, J.; Baldomà, L.; Calpena, A.C.; Biancardi, M.; Souto, E.B.; García, M.L.; et al. Development of topical eye-drops of lactoferrin-loaded biodegradable nanoparticles for the treatment of anterior segment inflammatory processes. Int. J. Pharm. 2021, 609, 121188.
- Varela-Fernández, R.; García-Otero, X.; Díaz-Tomé, V.; Regueiro, U.; López-López, M.; González-Barcia, M.; Isabel Lema, M.; Otero-Espinar, F.J. Mucoadhesive PLGA Nanospheres and Nanocapsules for Lactoferrin Controlled Ocular Delivery. Pharmaceutics 2022, 14, 799.
- López-Machado, A.; Díaz-Garrido, N.; Cano, A.; Espina, M.; Badia, J.; Baldomà, L.; Calpena, A.C.; Souto, E.B.; García, M.L.; Sánchez-López, E. Development of Lactoferrin-Loaded Liposomes for the Management of Dry Eye Disease and Ocular Inflammation. Pharmaceutics 2021, 13, 1698.
- Varela-Fernández, R.; García-Otero, X.; Díaz-Tomé, V.; Regueiro, U.; López-López, M.; González-Barcia, M.; Isabel Lema, M.; Javier Otero-Espinar, F. Lactoferrin-loaded nanostructured lipid carriers (NLCs) as a new formulation for optimized ocular drug delivery. Eur. J. Pharm. Biopharm. 2022, 172, 144–156.
- He, W.; Guo, X.; Feng, M.; Mao, N. In vitro and in vivo studies on ocular vitamin A palmitate cationic liposomal in situ gels. Int. J. Pharm. 2013, 458, 305–314.
- Castro, B.F.M.; Fulgêncio, G.d.O.; Domingos, L.C.; Cotta, O.A.L.; Silva-Cunha, A.; Fialho, S.L. Positively charged polymeric nanoparticles improve ocular penetration of tacrolimus after topical administration. J. Drug Deliv. Sci. Technol. 2020, 60, 101912.
- Mohammad; Garg, V.; Nirmal, J.; Warsi, M.H.; Pandita, D.; Kesharwani, P.; Jain, G.K. Topical Tacrolimus Progylcosomes Nano-Vesicles As a Potential Therapy for Experimental Dry Eye Syndrome. J. Pharm. Sci. 2022, 111, 479–484.
- Zhang, C.; Zheng, Y.; Li, M.; Zhang, Z.; Chang, L.; Ai, M.; Wang, J.; Zhao, S.; Li, C.; Zhou, Z. Carboxymethyl Cellulose-Coated Tacrolimus Nonspherical Microcrystals for Improved Therapeutic Efficacy of Dry Eye. Macromol. Biosci. 2020, 20, 2000079.
- Chen, X.; Wu, J.; Lin, X.; Wu, X.; Yu, X.; Wang, B.; Xu, W. Tacrolimus Loaded Cationic Liposomes for Dry Eye Treatment. Front. Pharmacol. 2022, 13, 838168.
- Liu, D.; Wu, Q.; Chen, W.; Lin, H.; Zhu, Y.; Liu, Y.; Liang, H.; Zhu, F. A novel FK506 loaded nanomicelles consisting of amino-terminated poly(ethylene glycol)-block-poly(D,L)-lactic acid and hydroxypropyl methylcellulose for ocular drug delivery. Int. J. Pharm. 2019, 562, 1–10.
- Bian, F.; Shin, C.S.; Wang, C.; Pflugfelder, S.C.; Acharya, G.; De Paiva, C.S. Dexamethasone Drug Eluting Nanowafers Control Inflammation in Alkali-Burned Corneas Associated with Dry Eye. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3222–3230.
- Tan, G.; Yu, S.; Li, J.; Pan, W. Development and characterization of nanostructured lipid carriers based chitosan thermosensitive hydrogel for delivery of dexamethasone. Int. J. Biol. Macromol. 2017, 103, 941–947.
- Taheri, S.L.; Rezazadeh, M.; Hassanzadeh, F.; Akbari, V.; Dehghani, A.; Talebi, A.; Mostafavi, S.A. Preparation, physicochemical, and retinal anti-angiogenic evaluation of poloxamer hydrogel containing dexamethasone/avastin-loaded chitosan-N-acetyl-L-cysteine nanoparticles. Int. J. Biol. Macromol. 2022, 220, 1605–1618.
- Alami-Milani, M.; Zakeri-Milani, P.; Valizadeh, H.; Sattari, S.; Salatin, S.; Jelvehgari, M. Evaluation of anti-inflammatory impact of dexamethasone-loaded PCL-PEG-PCL micelles on endotoxin-induced uveitis in rabbits. Pharm. Dev. Technol. 2019, 24, 680–688.
- Kassem, M.A.; Abdel Rahman, A.A.; Ghorab, M.M.; Ahmed, M.B.; Khalil, R.M. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int. J. Pharm. 2007, 340, 126–133.
- Fialho, S.L.; Da Silva-Cunha, A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin. Exp. Ophthalmol. 2004, 32, 626–632.
- Swaminathan, S.; Vavia, P.R.; Trotta, F.; Cavalli, R. Nanosponges Encapsulating Dexamethasone for Ocular Delivery: Formulation Design, Physicochemical Characterization, Safety and Corneal Permeability Assessment. J. Biomed. Nanotechnol. 2013, 9, 998–1007.
- Wang, T.-Z.; Guan, B.; Liu, X.-X.; Ke, L.-N.; Wang, J.-J.; Nan, K.-H. A topical fluorometholone nanoformulation fabricated under aqueous condition for the treatment of dry eye. Colloids Surf. B Biointerfaces 2022, 212, 112351.
- Safwat, M.A.; Mansour, H.F.; Hussein, A.K.; Abdelwahab, S.; Soliman, G.M. Polymeric micelles for the ocular delivery of triamcinolone acetonide: Preparation and in vivo evaluation in a rabbit ocular inflammatory model. Drug Deliv. 2020, 27, 1115–1124.
- Sabzevari, A.; Adibkia, K.; Hashemi, H.; De Geest, B.G.; Mohsenzadeh, N.; Atyabi, F.; Ghahremani, M.H.; Khoshayand, M.-R.; Dinarvand, R. Improved Anti-Inflammatory Effects in Rabbit Eye Model Using Biodegradable Poly Beta-Amino Ester Nanoparticles of Triamcinolone Acetonide. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5520–5526.
- Zimmer, A.K.; Maincent, P.; Thouvenot, P.; Kreuter, J. Hydrocortisone delivery to healthy and inflamed eyes using a micellar polysorbate 80 solution or albumin nanoparticles. Int. J. Pharm. 1994, 110, 211–222.
- Ibrahim, H.K.; El-Leithy, I.S.; Makky, A.A. Mucoadhesive Nanoparticles as Carrier Systems for Prolonged Ocular Delivery of Gatifloxacin/Prednisolone Bitherapy. Mol. Pharm. 2010, 7, 576–585.
- Schopf, L.; Enlow, E.; Popov, A.; Bourassa, J.; Chen, H. Ocular Pharmacokinetics of a Novel Loteprednol Etabonate 0.4% Ophthalmic Formulation. Ophthalmol. Ther. 2014, 3, 63–72.
- Asasutjarit, R.; Theerachayanan, T.; Kewsuwan, P.; Veeranodha, S.; Fuongfuchat, A.; Ritthidej, G.C. Development and Evaluation of Diclofenac Sodium Loaded-N-Trimethyl Chitosan Nanoparticles for Ophthalmic Use. AAPS PharmSciTech 2015, 16, 1013–1024.
- Agnihotri, S.M.; Vavia, P.R. Diclofenac-loaded biopolymeric nanosuspensions for ophthalmic application. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 90–95.
- Abrego, G.; Alvarado, H.L.; Egea, M.A.; Gonzalez-Mira, E.; Calpena, A.C.; Garcia, M.L. Design of Nanosuspensions and Freeze-Dried PLGA Nanoparticles as a Novel Approach for Ophthalmic Delivery of Pranoprofen. J. Pharm. Sci. 2014, 103, 3153–3164.
- Luo, Y.; Yang, L.; Feng, P.; Qiu, H.; Wu, X.; Lu, S.; Zhou, M.; Xu, L.; Zhu, Y. Pranoprofen Nanoparticles with Poly(L-Lactide)-b-Poly(Ethylene Glycol)-b-Poly(L-Lactide) as the Matrix Toward Improving Ocular Anti-inflammation. Front. Bioeng. Biotechnol. 2020, 8, 581621.
- Sánchez-Santos, I.; García-Sánchez, G.A.; Gonzalez-Salinas, R.; Linares-Alba, M.A.; Rodríguez-Reyes, A.A.; García-Santisteban, R.; Tirado-González, V.; Hernández-Piñamora, E.; García-Arzate, D.; Morales-Cantón, V.; et al. Intravitreal bromfenac liposomal suspension (100 μg/0.1 mL). A safety study in rabbit eyes. Exp. Eye Res. 2020, 194, 108020.
- Otake, H.; Goto, R.; Ogata, F.; Isaka, T.; Kawasaki, N.; Kobayakawa, S.; Matsunaga, T.; Nagai, N. Fixed-Combination Eye Drops Based on Fluorometholone Nanoparticles and Bromfenac/Levofloxacin Solution Improve Drug Corneal Penetration. Int. J. Nanomed. 2021, 16, 5343–5356.
- Shoman, N.A.; Gebreel, R.M.; El-Nabarawi, M.A.; Attia, A. Optimization of hyaluronan-enriched cubosomes for bromfenac delivery enhancing corneal permeation: Characterization, ex vivo, and in vivo evaluation. Drug Deliv. 2023, 30, 2162162.
- Warsi, M.H. Development and optimization of vitamin E TPGS based PLGA nanoparticles for improved and safe ocular delivery of ketorolac. J. Drug Deliv. Sci. Technol. 2021, 61, 102121.
- Tauber, J.; Karpecki, P.; Latkany, R.; Luchs, J.; Martel, J.; Sall, K.; Raychaudhuri, A.; Smith, V.; Semba, C.P. Lifitegrast Ophthalmic Solution 5.0% versus Placebo for Treatment of Dry Eye Disease: Results of the Randomized Phase III OPUS-2 Study. Ophthalmology 2015, 122, 2423–2431.
- Hovanesian, J.; Epitropoulos, A.; Donnenfeld, E.D.; Holladay, J.T. The Effect of Lifitegrast on Refractive Accuracy and Symptoms in Dry Eye Patients Undergoing Cataract Surgery. Clin. Ophthalmol. (Auckl. N.Z.) 2020, 14, 2709–2716.
- Cheng, Y.-H.; Ko, Y.-C.; Chang, Y.-F.; Huang, S.-H.; Liu, C.J.-l. Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded nanoparticles and latanoprost as a dual-drug delivery system for glaucoma treatment. Exp. Eye Res. 2019, 179, 179–187.
- Dang, H.; Dong, C.; Zhang, L. Sustained latanoprost release from PEGylated solid lipid nanoparticle-laden soft contact lens to treat glaucoma. Pharm. Dev. Technol. 2022, 27, 127–133.
- Xu, J.; Ge, Y.; Bu, R.; Zhang, A.; Feng, S.; Wang, J.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; et al. Co-delivery of latanoprost and timolol from micelles-laden contact lenses for the treatment of glaucoma. J. Control. Release 2019, 305, 18–28.
- Bessone, C.D.V.; Akhlaghi, S.P.; Tártara, L.I.; Quinteros, D.A.; Loh, W.; Allemandi, D.A. Latanoprost-loaded phytantriol cubosomes for the treatment of glaucoma. Eur. J. Pharm. Sci. 2021, 160, 105748.
- Schnichels, S.; Hurst, J.; de Vries, J.W.; Ullah, S.; Gruszka, A.; Kwak, M.; Löscher, M.; Dammeier, S.; Bartz-Schmidt, K.-U.; Spitzer, M.S.; et al. Self-assembled DNA nanoparticles loaded with travoprost for glaucoma-treatment. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102260.
- Masse, F.; Ouellette, M.; Boisselier, E. Ultrastable gold nanoparticles as a new drug vector for glaucoma therapy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3512.
- Shukr, M.H.; Ismail, S.; El-Hossary, G.G.; El-Shazly, A.H. Design and evaluation of mucoadhesive in situ liposomal gel for sustained ocular delivery of travoprost using two steps factorial design. J. Drug Deliv. Sci. Technol. 2021, 61, 102333.
- Shukr, M.H.; Ismail, S.; El-Hossary, G.G.; El-Shazly, A.H. Spanlastics nanovesicular ocular insert as a novel ocular delivery of travoprost: Optimization using Box–Behnken design and in vivo evaluation. J. Liposome Res. 2022, 32, 354–364.
- Ismail, A.; Nasr, M.; Sammour, O. Nanoemulsion as a feasible and biocompatible carrier for ocular delivery of travoprost: Improved pharmacokinetic/pharmacodynamic properties. Int. J. Pharm. 2020, 583, 119402.
- Goldstein, M.H.; Goldberg, D.; Walters, T.R.; Vantipalli, S.; Braun, E.; Metzinger, J.L. Evaluating Safety, Tolerability and Efficacy of an Intracameral Hydrogel-Based Travoprost Implant in Subjects with Glaucoma-Phase 1 Trial. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4266.
- Li, Q.; Ma, C.; Ma, Y.; Ma, Y.; Mao, Y.; Meng, Z. Sustained bimatoprost release using gold nanoparticles laden contact lenses. J. Biomater. Sci. Polym. Ed. 2021, 32, 1618–1634.
- Meany, E.L.; Andaya, R.; Tang, S.; Kasse, C.M.; Fuji, R.N.; Grosskopf, A.K.; d’Aquino, A.L.; Bartoe, J.T.; Ybarra, R.; Shelton, A.; et al. Injectable Polymer-Nanoparticle Hydrogel for the Sustained Intravitreal Delivery of Bimatoprost. Adv. Ther. 2023, 6, 2200207.
- Xu, W.; Jiao, W.; Li, S.; Tao, X.; Mu, G. Bimatoprost loaded microemulsion laden contact lens to treat glaucoma. J. Drug Deliv. Sci. Technol. 2019, 54, 101330.
- Maulvi, F.A.; Soni, P.D.; Patel, P.J.; Desai, A.R.; Desai, D.T.; Shukla, M.R.; Shah, S.A.; Shah, D.O.; Willcox, M.D.P. Controlled bimatoprost release from graphene oxide laden contact lenses: In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2021, 208, 112096.
- Seal, J.R.; Robinson, M.R.; Burke, J.; Bejanian, M.; Coote, M.; Attar, M. Intracameral Sustained-Release Bimatoprost Implant Delivers Bimatoprost to Target Tissues with Reduced Drug Exposure to Off-Target Tissues. J. Ocul. Pharmacol. Ther. 2019, 35, 50–57.
- Yadav, M.; Guzman-Aranguez, A.; Perez de Lara, M.J.; Singh, M.; Singh, J.; Kaur, I.P. Bimatoprost loaded nanovesicular long-acting sub-conjunctival in-situ gelling implant: In vitro and in vivo evaluation. Mater. Sci. Eng. C 2019, 103, 109730.
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Rodrigues, L.B.; Bravo, R.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.J.P.O. Bimatoprost-loaded ocular inserts as sustained release drug delivery systems for glaucoma treatment: In vitro and in vivo evaluation. PLoS ONE 2014, 9, e95461.
- Nagai, N.; Yamada, S.; Kawasaki, J.; Koyanagi, E.; Saijo, S.; Kaji, H.; Nishizawa, M.; Nakazawa, T.; Abe, T. Pharmacokinetic and Safety Evaluation of a Transscleral Sustained Unoprostone Release Device in Monkey Eyes. Investig. Ophthalmol. Vis. Sci. 2018, 59, 644–652.
- Khallaf, A.M.; El-Moslemany, R.M.; Ahmed, M.F.; Morsi, M.H.; Khalafallah, N.M. Exploring a Novel Fasudil-Phospholipid Complex Formulated as Liposomal Thermosensitive in situ Gel for Glaucoma. Int. J. Nanomed. 2022, 17, 163–181.
- Mietzner, R.; Kade, C.; Froemel, F.; Pauly, D.; Stamer, W.D.; Ohlmann, A.; Wegener, J.; Fuchshofer, R.; Breunig, M. Fasudil Loaded PLGA Microspheres as Potential Intravitreal Depot Formulation for Glaucoma Therapy. Pharmaceutics 2020, 12, 706.
- Kusuhara, S.; Nakamura, M. Ripasudil Hydrochloride Hydrate in the Treatment of Glaucoma: Safety, Efficacy, and Patient Selection. Clin. Ophthalmol. 2020, 14, 1229–1236.
- Mehran, N.A.; Sinha, S.; Razeghinejad, R. New glaucoma medications: Latanoprostene bunod, netarsudil, and fixed combination netarsudil-latanoprost. Eye 2020, 34, 72–88.
- Jung, H.J.; Abou-Jaoude, M.; Carbia, B.E.; Plummer, C.; Chauhan, A. Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses. J. Control. Release 2013, 165, 82–89.
- Cuggino, J.C.; Tártara, L.I.; Gugliotta, L.M.; Palma, S.D.; Alvarez Igarzabal, C.I. Mucoadhesive and responsive nanogels as carriers for sustainable delivery of timolol for glaucoma therapy. Mater. Sci. Eng. C 2021, 118, 111383.
- Hathout, R.M.; Gad, H.A.; Abdel-Hafez, S.M.; Nasser, N.; Khalil, N.; Ateyya, T.; Amr, A.; Yasser, N.; Nasr, S.; Metwally, A.A. Gelatinized core liposomes: A new Trojan horse for the development of a novel timolol maleate glaucoma medication. Int. J. Pharm. 2019, 556, 192–199.
- Wei, N.; Dang, H.; Huang, C.; Sheng, Y. Timolol loaded microemulsion laden silicone contact lens to manage glaucoma: In vitro and in vivo studies. J. Dispers. Sci. Technol. 2021, 42, 742–750.
- Kumar, N.; Aggarwal, R.; Chauhan, M.K. Extended levobunolol release from Eudragit nanoparticle-laden contact lenses for glaucoma therapy. Future J. Pharm. Sci. 2020, 6, 109.
- Karataş, A.; Sonakin, Ö.; KiliÇarslan, M.; Baykara, T. Poly (ε-caprolactone) microparticles containing Levobunolol HCl prepared by a multiple emulsion (W/O/W) solvent evaporation technique: Effects of some formulation parameters on microparticle characteristics. J. Microencapsul. 2009, 26, 63–74.
- Marchal-Heussler, L.; Sirbat, D.; Hoffman, M.; Maincent, P. Poly(ε-Caprolactone) Nanocapsules in Carteolol Ophthalmic Delivery. Pharm. Res. 1993, 10, 386–390.
- Nagai, N.; Yamaoka, S.; Fukuoka, Y.; Ishii, M.; Otake, H.; Kanai, K.; Okamoto, N.; Shimomura, Y. Enhancement in Corneal Permeability of Dissolved Carteolol by Its Combination with Magnesium Hydroxide Nanoparticles. Int. J. Mol. Sci. 2018, 19, 282.
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Alharbi, K.S.; Yasir, M.; Elmowafy, M.; Ansari, M.J.; Salahuddin, M.; Alshehri, S. Formulation of carteolol chitosomes for ocular delivery: Formulation optimization, ex-vivo permeation, and ocular toxicity examination. Cutan. Ocul. Toxicol. 2021, 40, 338–349.
- Losa, C.; Marchal-Heussler, L.; Orallo, F.; Jato, J.L.V.; Alonso, M.J. Design of New Formulations for Topical Ocular Administration: Polymeric Nanocapsules Containing Metipranolol. Pharm. Res. 1993, 10, 80–87.
- Huang, Y.; Tao, Q.; Hou, D.; Hu, S.; Tian, S.; Chen, Y.; Gui, R.; Yang, L.; Wang, Y. A novel ion-exchange carrier based upon liposome-encapsulated montmorillonite for ophthalmic delivery of betaxolol hydrochloride. Int. J. Nanomed. 2017, 12, 1731–1745.
- Zhao, Y.; Li, J.; Han, X.; Tao, Q.; Liu, S.; Jiang, G.; Zhu, G.; Yang, F.; Lv, Z.; Chen, Y.; et al. Dual controlled release effect of montmorillonite loaded polymer nanoparticles for ophthalmic drug delivery. Appl. Clay Sci. 2019, 180, 105167.
- Allam, A.; Elsabahy, M.; El Badry, M.; Eleraky, N.E. Betaxolol-loaded niosomes integrated within pH-sensitive in situ forming gel for management of glaucoma. Int. J. Pharm. 2021, 598, 120380.
- Sun, J.; Lei, Y.; Dai, Z.; Liu, X.; Huang, T.; Wu, J.; Xu, Z.P.; Sun, X. Sustained Release of Brimonidine from a New Composite Drug Delivery System for Treatment of Glaucoma. ACS Appl. Mater. Interfaces 2017, 9, 7990–7999.
- Shivakumar, H.N.; Desai, B.G.; Subhash, P.G.; Ashok, P.; Hulakoti, B. Design of ocular inserts of brimonidine tartrate by response surface methodology. J. Drug Deliv. Sci. Technol. 2007, 17, 421–430.
- Emad Eldeeb, A.; Salah, S.; Ghorab, M. Proniosomal gel-derived niosomes: An approach to sustain and improve the ocular delivery of brimonidine tartrate; formulation, in-vitro characterization, and in-vivo pharmacodynamic study. Drug Deliv. 2019, 26, 509–521.
- Chiang, B.; Kim, Y.C.; Doty, A.C.; Grossniklaus, H.E.; Schwendeman, S.P.; Prausnitz, M.R. Sustained reduction of intraocular pressure by supraciliary delivery of brimonidine-loaded poly(lactic acid) microspheres for the treatment of glaucoma. J. Control. Release 2016, 228, 48–57.
- Bigdeli, A.; Makhmalzadeh, B.S.; Feghhi, M.; SoleimaniBiatiani, E. Cationic liposomes as promising vehicles for timolol/brimonidine combination ocular delivery in glaucoma: Formulation development and in vitro/in vivo evaluation. Drug Deliv. Transl. Res. 2022, 13, 1035–1047.
- Zhao, Y.; Huang, C.; Zhang, Z.; Hong, J.; Xu, J.; Sun, X.; Sun, J. Sustained release of brimonidine from @TPU implant for treatment of glaucoma. Drug Deliv. 2022, 29, 613–623.
- Abdel Azim, E.A.; Elkheshen, S.A.; Hathout, R.M.; Fouly, M.A.; El Hoffy, N.M. Augmented in vitro and in vivo Profiles of Brimonidine Tartrate Using Gelatinized-Core Liposomes. Int. J. Nanomed. 2022, 17, 2753–2776.
- Shahab, M.S.; Rizwanullah, M.; Alshehri, S.; Imam, S.S. Optimization to development of chitosan decorated polycaprolactone nanoparticles for improved ocular delivery of dorzolamide: In vitro, ex vivo and toxicity assessments. Int. J. Biol. Macromol. 2020, 163, 2392–2404.
- Kassem, A.A.; Salama, A.; Mohsen, A.M. Formulation and optimization of cationic nanoemulsions for enhanced ocular delivery of dorzolamide hydrochloride using Box-Behnken design: In vitro and in vivo assessments. J. Drug Deliv. Sci. Technol. 2022, 68, 103047.
- Kouchak, M.; Malekahmadi, M.; Bavarsad, N.; Saki Malehi, A.; Andishmand, L. Dorzolamide nanoliposome as a long action ophthalmic delivery system in open angle glaucoma and ocular hypertension patients. Drug Dev. Ind. Pharm. 2018, 44, 1239–1242.
- Fu, J.; Sun, F.; Liu, W.; Liu, Y.; Gedam, M.; Hu, Q.; Fridley, C.; Quigley, H.A.; Hanes, J.; Pitha, I. Subconjunctival Delivery of Dorzolamide-Loaded Poly(ether-anhydride) Microparticles Produces Sustained Lowering of Intraocular Pressure in Rabbits. Mol. Pharm. 2016, 13, 2987–2995.
- Fouda, N.H.; Abdelrehim, R.T.; Hegazy, D.A.; Habib, B.A. Sustained ocular delivery of Dorzolamide-HCl via proniosomal gel formulation: In-vitro characterization, statistical optimization, and in-vivo pharmacodynamic evaluation in rabbits. Drug Deliv. 2018, 25, 1340–1349.
- Özdemir, S.; Çakırlı, E.; Sürücü, B.; Aygüler, C.İ.; Üner, B.; Çelebi, A.R.C. Preparation and characterization studies of dorzolamide loaded ophthalmic implants for the treatment of glaucoma. Turk. J. Pharm. Sci. 2022.
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.A.; Cronemberger, S.; Nogueira, J.C.; et al. Chitosan/hydroxyethyl cellulose inserts for sustained-release of dorzolamide for glaucoma treatment: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 570, 118662.
- Wu, W.; Li, J.; Wu, L.; Wang, B.; Wang, Z.; Xu, Q.; Xin, H. Ophthalmic Delivery of Brinzolamide by Liquid Crystalline Nanoparticles: In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2013, 14, 1063–1071.
- Tuomela, A.; Liu, P.; Puranen, J.; Rönkkö, S.; Laaksonen, T.; Kalesnykas, G.; Oksala, O.; Ilkka, J.; Laru, J.; Järvinen, K.; et al. Brinzolamide nanocrystal formulations for ophthalmic delivery: Reduction of elevated intraocular pressure in vivo. Int. J. Pharm. 2014, 467, 34–41.
- Li, H.; Liu, Y.; Zhang, Y.; Fang, D.; Xu, B.; Zhang, L.; Chen, T.; Ren, K.; Nie, Y.; Yao, S.; et al. Liposomes as a Novel Ocular Delivery System for Brinzolamide: In Vitro and In Vivo Studies. AAPS PharmSciTech 2016, 17, 710–717.
- Bhalerao, H.; Koteshwara, K.B.; Chandran, S. Design, optimisation and evaluation of in situ gelling nanoemulsion formulations of brinzolamide. Drug Deliv. Transl. Res. 2020, 10, 529–547.
- Cegielska, O.; Sierakowski, M.; Sajkiewicz, P.; Lorenz, K.; Kogermann, K. Mucoadhesive brinzolamide-loaded nanofibers for alternative glaucoma treatment. Eur. J. Pharm. Biopharm. 2022, 180, 48–62.
- Smith, S.M.; Salmon, J.H.; Abbaraju, S.; Amin, R.; Gilger, B.C. Tolerability, pharmacokinetics, and pharmacodynamics of a brinzolamide episcleral sustained release implant in normotensive New Zealand white rabbits. J. Drug Deliv. Sci. Technol. 2021, 61, 102123.
- Teba, H.E.; Khalil, I.A.; El Sorogy, H.M. Novel cubosome based system for ocular delivery of acetazolamide. Drug Deliv. 2021, 28, 2177–2186.
- Abdel-Rashid, R.S.; Helal, D.A.; Omar, M.M.; El Sisi, A.M. Nanogel loaded with surfactant based nanovesicles for enhanced ocular delivery of acetazolamide. Int. J. Nanomed. 2019, 14, 2973–2983.
- Mazyed, E.A.; Abdelaziz, A.E. Fabrication of Transgelosomes for Enhancing the Ocular Delivery of Acetazolamide: Statistical Optimization, In Vitro Characterization, and In Vivo Study. Pharmaceutics 2020, 12, 465.
- Morais, M.; Coimbra, P.; Pina, M.E. Comparative Analysis of Morphological and Release Profiles in Ocular Implants of Acetazolamide Prepared by Electrospinning. Pharmaceutics 2021, 13, 260.
- El-Menshawe, S.F. A novel approach to topical acetazolamide/PEG 400 ocular niosomes. J. Drug Deliv. Sci. Technol. 2012, 22, 295–299.
- Obiedallah, M.M.; Abdel-Mageed, A.M.; Elfaham, T.H. Ocular administration of acetazolamide microsponges in situ gel formulations. Saudi Pharm. J. 2018, 26, 909–920.
- Mishra, V.; Jain, N.K. Acetazolamide encapsulated dendritic nano-architectures for effective glaucoma management in rabbits. Int. J. Pharm. 2014, 461, 380–390.
- Lin, H.-R.; Yu, S.-P.; Kuo, C.-J.; Kao, H.-J.; Lo, Y.-L.; Lin, Y.-J. Pilocarpine-loaded chitosan-PAA nanosuspension for ophthalmic delivery. J. Biomater. Sci. Polym. Ed. 2007, 18, 205–221.
- Suketu, D.; Desai, J.B. Pluronic® F127-Based Ocular Delivery System Containing Biodegradable Polyisobutylcyanoacrylate Nanocapsules of Pilocarpine. Drug Deliv. 2000, 7, 201–207.
- Patel, C.C.; Mandava, N.; Oliver, S.C.N.; Braverman, R.; Quiroz-Mercado, H.; Olson, J.L. Treatment of Intractable Posterior Uveitis in Pediatric Patients with the Fluocinolone Acetonide Intravitreal Implant (Retisert). J. Retin. Vitr. Dis. 2012, 32, 537–542.
- Salama, A.H.; Mahmoud, A.A.; Kamel, R. A Novel Method for Preparing Surface-Modified Fluocinolone Acetonide Loaded PLGA Nanoparticles for Ocular Use: In Vitro and In Vivo Evaluations. AAPS PharmSciTech 2016, 17, 1159–1172.
- Shelley, H.; Annaji, M.; Grant, M.; Fasina, O.; Babu, R.J. Sustained Release Biodegradable Microneedles of Difluprednate for Delivery to Posterior Eye. J. Ocul. Pharmacol. Ther. 2022, 38, 449–458.
- Gonzalez-Pizarro, R.; Parrotta, G.; Vera, R.; Sánchez-López, E.; Galindo, R.; Kjeldsen, F.; Badia, J.; Baldoma, L.; Espina, M.; García, M.L. Ocular penetration of fluorometholone-loaded PEG-PLGA nanoparticles functionalized with cell-penetrating peptides. Nanomedicine 2019, 14, 3089–3104.
- Baba, K.; Hashida, N.; Tujikawa, M.; Quantock, A.J.; Nishida, K. The generation of fluorometholone nanocrystal eye drops, their metabolization to dihydrofluorometholone and penetration into rabbit eyes. Int. J. Pharm. 2021, 592, 120067.
- Nirbhavane, P.; Sharma, G.; Singh, B.; Begum, G.; Jones, M.-C.; Rauz, S.; Vincent, R.; Denniston, A.K.; Hill, L.J.; Katare, O.P. Triamcinolone acetonide loaded-cationic nano-lipoidal formulation for uveitis: Evidences of improved biopharmaceutical performance and anti-inflammatory activity. Colloids Surf. B Biointerfaces 2020, 190, 110902.
- Chen, Z.; Yang, M.; Wang, Q.; Bai, J.; McAlinden, C.; Skiadaresi, E.; Zhang, J.; Pan, L.; Mei, C.; Zeng, Z.; et al. Hydrogel eye drops as a non-invasive drug carrier for topical enhanced Adalimumab permeation and highly efficient uveitis treatment. Carbohydr. Polym. 2021, 253, 117216.
- Zhang, R.; Qian, J.; Li, X.; Yuan, Y. Treatment of experimental autoimmune uveoretinitis with intravitreal injection of infliximab encapsulated in liposomes. Br. J. Ophthalmol. 2017, 101, 1731–1738.
- Manna, S.; Donnell, A.M.; Faraj, R.Q.C.; Riemann, B.I.; Riemann, C.D.; Augsburger, J.J.; Correa, Z.M.; Banerjee, R.K. Pharmacokinetics and Toxicity Evaluation of a PLGA and Chitosan-Based Micro-Implant for Sustained Release of Methotrexate in Rabbit Vitreous. Pharmaceutics 2021, 13, 1227.
- Paiva, M.R.B.D.; Vasconcelos-Santos, D.V.; Vieira, L.C.; Fialho, S.L.; Silva-Cunha, A. Sirolimus-Loaded Intravitreal Implant for Effective Treatment of Experimental Uveitis. AAPS PharmSciTech 2021, 22, 35.
- Wu, W.; He, Z.; Zhang, Z.; Yu, X.; Song, Z.; Li, X. Intravitreal injection of rapamycin-loaded polymeric micelles for inhibition of ocular inflammation in rat model. Int. J. Pharm. 2016, 513, 238–246.
- Li, H.; Zhang, Z.; Li, Y.; Su, L.; Duan, Y.; Zhang, H.; An, J.; Ni, T.; Li, X.; Zhang, X. Therapeutic Effect of Rapamycin-Loaded Small Extracellular Vesicles Derived from Mesenchymal Stem Cells on Experimental Autoimmune Uveitis. Front. Immunol. 2022, 13, 864956.
- Silva, N.C.; Silva, S.; Sarmento, B.; Pintado, M. Chitosan nanoparticles for daptomycin delivery in ocular treatment of bacterial endophthalmitis. Drug Deliv. 2015, 22, 885–893.
- Jounaki, K.; Makhmalzadeh, B.S.; Feghhi, M.; Heidarian, A. Topical ocular delivery of vancomycin loaded cationic lipid nanocarriers as a promising and non-invasive alternative approach to intravitreal injection for enhanced bacterial endophthalmitis management. Eur. J. Pharm. Sci. 2021, 167, 105991.
- Cardoso, J.F.; Perasoli, F.B.; Almeida, T.C.; Marques, M.B.D.F.; Toledo, C.R.; Gil, P.O.; Tavares, H.D.S.; Da Paz, M.C.; Mussel, W.D.N.; Magalhães, J.T.; et al. Vancomycin-loaded N,N-dodecyl,methyl-polyethylenimine nanoparticles coated with hyaluronic acid to treat bacterial endophthalmitis: Development, characterization, and ocular biocompatibility. Int. J. Biol. Macromol. 2021, 169, 330–341.
- Dosmar, E.; Liu, W.; Patel, G.; Rogozinski, A.; Mieler, W.F.; Kang-Mieler, J.J. Controlled Release of Vancomycin From a Thermoresponsive Hydrogel System for the Prophylactic Treatment of Postoperative Acute Endophthalmitis. Transl. Vis. Sci. Technol. 2019, 8, 53.
- Abrishami, M.; Motamed Shariati, M.; Malaekeh-Nikouei, B.; Tajani, A.S.; Mahmoudi, A.; Abrishami, M.; Khameneh, B. Preparation and in vivo evaluation of nanoliposomes containing vancomycin after intravitreal injection in albino rabbits. Iran. J. Basic Med. Sci. 2020, 23, 551–555.
- Resende, A.F.C.; Pereira, A.F.; Moreira, T.P.; Patrício, P.S.O.; Fialho, S.L.; Cunha, G.M.F.; Silva-Cunha, A.; Magalhães, J.T.; Silva, G.R. PLGA Implants containing vancomycin and dexamethasone: Development, characterization and bactericidal effects. Pharm.-Int. J. Pharm. Sci. 2016, 71, 439–446.
- Allam, A.; El-Mokhtar, M.A.; Elsabahy, M. Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for treatment of ocular infections while minimizing drug irritation. J. Pharm. Pharmacol. 2019, 71, 1209–1221.
- Mohammadpour, M.; Jabbarvand, M.; Karimi, N. Therapeutic possibilities of ceftazidime nanoparticles in devastating pseudomonas ophthalmic infections; keratitis and endophthalmitis. Med. Hypothesis Discov. Innov. Ophthalmol. J. 2012, 1, 6–9.
- Bae, J.H.; Lee, S.C. Intravitreal liposomal amphotericin B for treatment of endogenous candida endophthalmitis. Jpn. J. Ophthalmol. 2015, 59, 346–352.
- Üstündağ Okur, N.; Yozgatlı, V.; Okur, M.E.; Yoltaş, A.; Siafaka, P.I. Improving therapeutic efficacy of voriconazole against fungal keratitis: Thermo-sensitive in situ gels as ophthalmic drug carriers. J. Drug Deliv. Sci. Technol. 2019, 49, 323–333.
- Kumar, R.; Sinha, V.R. Solid lipid nanoparticle: An efficient carrier for improved ocular permeation of voriconazole. Drug Dev. Ind. Pharm. 2016, 42, 1956–1967.
- Bhosale, R.; Bhandwalkar, O.; Duduskar, A.; Jadhav, R.; Pawar, P. Water soluble chitosan mediated voriconazole microemulsion as sustained carrier for ophthalmic application: In vitro/ex vivo/in vivo evaluations. Open Pharm. Sci. J. 2016, 3, 215–234.
- Fahmy, A.M.; Hassan, M.; El-Setouhy, D.A.; Tayel, S.A.; Al-mahallawi, A.M. Voriconazole Ternary Micellar Systems for the Treatment of Ocular Mycosis: Statistical Optimization and In Vivo Evaluation. J. Pharm. Sci. 2021, 110, 2130–2138.
- de Sá, F.A.P.; Taveira, S.F.; Gelfuso, G.M.; Lima, E.M.; Gratieri, T. Liposomal voriconazole (VOR) formulation for improved ocular delivery. Colloids Surf. B Biointerfaces 2015, 133, 331–338.
- Ma, F.; Nan, K.; Lee, S.; Beadle, J.R.; Hou, H.; Freeman, W.R.; Hostetler, K.Y.; Cheng, L. Micelle formulation of hexadecyloxypropyl-cidofovir (HDP-CDV) as an intravitreal long-lasting delivery system. Eur. J. Pharm. Biopharm. 2015, 89, 271–279.
- Kuppermann, B.D.; Assil, K.K.; Vuong, C.; Besen, G.; Wiley, C.A.; De Clercq, E.; Bergeron-Lynn, G.; Connor, J.D.; Pursley, M.; Munguia, D.; et al. Liposome-Encapsulated (S)-1-(3-Hydroxy-2-Phosphonylmethoxypropyl)cytosine for Long-Acting Therapy of Viral Retinitis. J. Infect. Dis. 1996, 173, 18–23.
- Claro, C.; Ruiz, R.; Cordero, E.; Pastor, M.T.; López-Cortés, L.F.; Jiménez-Castellanos, M.R.; Lucero, M.J. Determination and pharmacokinetic profile of liposomal foscarnet in rabbit ocular tissues after intravitreal administration. Exp. Eye Res. 2009, 88, 528–534.
- Peter, S.; Mathews, M.M.; Saju, F.; Paul, S. Development, Optimization and In Vitro Characterization of Eudragit-Ganciclovir Nanosuspension or Treating Herpes Simplex Keratitis. J. Pharm. Innov. 2023, 1–10.
- Naguib, M.J.; Hassan, Y.R.; Abd-Elsalam, W.H. 3D printed ocusert laden with ultra-fluidic glycerosomes of ganciclovir for the management of ocular cytomegalovirus retinitis. Int. J. Pharm. 2021, 607, 121010.
- Choudhari, M.; Nayak, K.; Nagai, N.; Nakazawa, Y.; Khunt, D.; Misra, M. Role of mucoadhesive agent in ocular delivery of ganciclovir microemulsion: Cytotoxicity evaluation in vitro and ex vivo. Int. Ophthalmol. 2022, 43, 1153–1167.
- Yasukawa, T.; Ogura, Y.; Kimura, H.; Sakurai, E.; Tabata, Y. Drug delivery from ocular implants. Expert Opin. Drug Deliv. 2006, 3, 261–273.
- Choonara, Y.E.; Pillay, V.; Carmichael, T.; Danckwerts, M.P. An in vitro study of the design and development of a novel doughnut-shaped minitablet for intraocular implantation. Int. J. Pharm. 2006, 310, 15–24.
- Yan, J.; Peng, X.; Cai, Y.; Cong, W. Development of facile drug delivery platform of ranibizumab fabricated PLGA-PEGylated magnetic nanoparticles for age-related macular degeneration therapy. J. Photochem. Photobiol. B Biol. 2018, 183, 133–136.
- Elsaid, N.; Jackson, T.L.; Elsaid, Z.; Alqathama, A.; Somavarapu, S. PLGA Microparticles Entrapping Chitosan-Based Nanoparticles for the Ocular Delivery of Ranibizumab. Mol. Pharm. 2016, 13, 2923–2940.
- Joseph, R.R.; Tan, D.W.N.; Ramon, M.R.M.; Natarajan, J.V.; Agrawal, R.; Wong, T.T.; Venkatraman, S.S. Characterization of liposomal carriers for the trans-scleral transport of Ranibizumab. Sci. Rep. 2017, 7, 16803.
- Qian, C.; Yan, P.; Wan, G.; Liang, S.; Dong, Y.; Wang, J. Facile synthetic Photoluminescent Graphene Quantum dots encapsulated β-cyclodextrin drug carrier system for the management of macular degeneration: Detailed analytical and biological investigations. J. Photochem. Photobiol. B Biol. 2018, 189, 244–249.
- Campochiaro, P.A.; Gune, S.; Maia, M.; Ding, H.T.; Maass, K. Pharmacokinetic profile of the Port Delivery System with ranibizumab (PDS) in the phase 3 Archway trial. Investig. Ophthalmol. Vis. Sci. 2021, 62, 350.
- Yandrapu, S.K.; Upadhyay, A.K.; Petrash, J.M.; Kompella, U.B. Nanoparticles in Porous Microparticles Prepared by Supercritical Infusion and Pressure Quench Technology for Sustained Delivery of Bevacizumab. Mol. Pharm. 2013, 10, 4676–4686.
- Jiang, P.; Chaparro, F.J.; Cuddington, C.T.; Palmer, A.F.; Ohr, M.P.; Lannutti, J.J.; Swindle-Reilly, K.E. Injectable biodegradable bi-layered capsule for sustained delivery of bevacizumab in treating wet age-related macular degeneration. J. Control. Release 2020, 320, 442–456.
- Zhang, X.-P.; Sun, J.-G.; Yao, J.; Shan, K.; Liu, B.-H.; Yao, M.-D.; Ge, H.-M.; Jiang, Q.; Zhao, C.; Yan, B. Effect of nanoencapsulation using poly (lactide-co-glycolide) (PLGA) on anti-angiogenic activity of bevacizumab for ocular angiogenesis therapy. Biomed. Pharmacother. 2018, 107, 1056–1063.
- Badiee, P.; Varshochian, R.; Rafiee-Tehrani, M.; Abedin Dorkoosh, F.; Khoshayand, M.R.; Dinarvand, R. Ocular implant containing bevacizumab-loaded chitosan nanoparticles intended for choroidal neovascularization treatment. J. Biomed. Mater. Res. 2018, 106, 2261–2271.
- Jiang, P.; Jacobs, K.M.; Ohr, M.P.; Swindle-Reilly, K.E. Chitosan–Polycaprolactone Core–Shell Microparticles for Sustained Delivery of Bevacizumab. Mol. Pharm. 2020, 17, 2570–2584.
- Karumanchi, D.K.; Skrypai, Y.; Thomas, A.; Gaillard, E.R. Rational design of liposomes for sustained release drug delivery of bevacizumab to treat ocular angiogenesis. J. Drug Deliv. Sci. Technol. 2018, 47, 275–282.
- Kelly, S.J.; Hirani, A.; Shahidadpury, V.; Solanki, A.; Halasz, K.; Varghese Gupta, S.; Madow, B.; Sutariya, V. Aflibercept Nanoformulation Inhibits VEGF Expression in Ocular In Vitro Model: A Preliminary Report. Biomedicines 2018, 6, 92.
- Liu, W.; Lee, B.-S.; Mieler, W.F.; Kang-Mieler, J.J. Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Bioactive Aflibercept In Vitro. Curr. Eye Res. 2019, 44, 264–274.
- Bhatt, P.; Narvekar, P.; Lalani, R.; Chougule, M.B.; Pathak, Y.; Sutariya, V. An in vitro Assessment of Thermo-Reversible Gel Formulation Containing Sunitinib Nanoparticles for Neovascular Age-Related Macular Degeneration. AAPS PharmSciTech 2019, 20, 281.
- Streets, J.; Bhatt, P.; Bhatia, D.; Sutariya, V. Sunitinib-Loaded MPEG-PCL Micelles for the Treatment of Age-Related Macular Degeneration. Sci. Pharm. 2020, 88, 30.
- Narvekar, P.; Bhatt, P.; Fnu, G.; Sutariya, V.J.A.; technologies, d.d. Axitinib-loaded poly (lactic-co-glycolic acid) nanoparticles for age-related macular degeneration: Formulation development and in vitro characterization. ASSAY Drug Dev. Technol. 2019, 17, 167–177.
- Eandi, C.M.; Alovisi, C.; De Sanctis, U.; Grignolo, F.M. Treatment for neovascular age related macular degeneration: The state of the art. Eur. J. Pharmacol. 2016, 787, 78–83.
- Chen, C.W.; Yeh, M.K.; Shiau, C.Y.; Chiang, C.H.; Lu, D.W. Efficient downregulation of VEGF in retinal pigment epithelial cells by integrin ligand-labeled liposome-mediated siRNA delivery. Int. J. Nanomed. 2013, 8, 2613–2627.
- Ryoo, N.K.; Lee, J.; Lee, H.; Hong, H.K.; Kim, H.; Lee, J.B.; Woo, S.J.; Park, K.H.; Kim, H. Therapeutic effects of a novel siRNA-based anti-VEGF (siVEGF) nanoball for the treatment of choroidal neovascularization. Nanoscale 2017, 9, 15461–15469.
- Wang, Z.; Liu, A.; Zhang, H.; Wang, M.; Tang, Q.; Huang, Y.; Wang, L. Inhibition of retinal neovascularization by VEGF siRNA delivered via bioreducible lipid-like nanoparticles. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2407–2418.
- Kim, H.; Csaky, K.G. Nanoparticle–integrin antagonist C16Y peptide treatment of choroidal neovascularization in rats. J. Control. Release 2010, 142, 286–293.
- Jayaraman, M.S.; Bharali, D.J.; Sudha, T.; Mousa, S.A. Nano chitosan peptide as a potential therapeutic carrier for retinal delivery to treat age-related macular degeneration. Mol. Vis. 2012, 18, 2300–2308.
- Nguyen, D.D.; Luo, L.-J.; Yang, C.-J.; Lai, J.-Y. Highly Retina-Permeating and Long-Acting Resveratrol/Metformin Nanotherapeutics for Enhanced Treatment of Macular Degeneration. ACS Nano 2023, 17, 168–183.
- Ayyagari, S.; Dar, H.; Morton, V.; Moy, K.; Patel, C.; Ramasubramanian, L.; Ravi, N.; Wood, S.; Zhao, A.; Zheng, M. Evaluation of Curcumin-Loaded Nanoliposomes for the Treatment and Prevention of Age-Related Macular Degeneration. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 2017.
- Sun, R.; Zhang, A.; Ge, Y.; Gou, J.; Yin, T.; He, H.; Wang, Y.; Zhang, G.; Kong, J.; Shang, L.; et al. Ultra-small-size Astragaloside-IV loaded lipid nanocapsules eye drops for the effective management of dry age-related macular degeneration. Expert Opin. Drug Deliv. 2020, 17, 1305–1320.
- Oh, E.J.; Choi, J.-S.; Kim, H.; Joo, C.-K.; Hahn, S.K. Anti-Flt1 peptide–Hyaluronate conjugate for the treatment of retinal neovascularization and diabetic retinopathy. Biomaterials 2011, 32, 3115–3123.
- Wang, C.; Seo, S.-J.; Kim, J.-S.; Lee, S.-H.; Jeon, J.-K.; Kim, J.-W.; Kim, K.-H.; Kim, J.-K.; Park, J. Intravitreal implantable magnetic micropump for on-demand VEGFR-targeted drug delivery. J. Control. Release 2018, 283, 105–112.
- Qiu, F.; Meng, T.; Chen, Q.; Zhou, K.; Shao, Y.; Matlock, G.; Ma, X.; Wu, W.; Du, Y.; Wang, X.; et al. Fenofibrate-Loaded Biodegradable Nanoparticles for the Treatment of Experimental Diabetic Retinopathy and Neovascular Age-Related Macular Degeneration. Mol. Pharm. 2019, 16, 1958–1970.
- Laddha, U.D.; Kshirsagar, S.J. Formulation of PPAR-gamma agonist as surface modified PLGA nanoparticles for non-invasive treatment of diabetic retinopathy: In vitro and in vivo evidences. Heliyon 2020, 6, e04589.
- Radwan, S.E.; El-Kamel, A.; Zaki, E.I.; Burgalassi, S.; Zucchetti, E.; El-Moslemany, R.M. Hyaluronic-Coated Albumin Nanoparticles for the Non-Invasive Delivery of Apatinib in Diabetic Retinopathy. Int. J. Nanomed. 2021, 16, 4481–4494.
- Jo, D.H.; Kim, J.H.; Yu, Y.S.; Lee, T.G.; Kim, J.H. Antiangiogenic effect of silicate nanoparticle on retinal neovascularization induced by vascular endothelial growth factor. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 784–791.
- Paiva, M.R.B.; Andrade, G.F.; Dourado, L.F.N.; Castro, B.F.M.; Fialho, S.L.; Sousa, E.M.B.; Silva-Cunha, A. Surface functionalized mesoporous silica nanoparticles for intravitreal application of tacrolimus. J. Biomater. Appl. 2021, 35, 1019–1033.
- Dave, V.; Sharma, R.; Gupta, C.; Sur, S. Folic acid modified gold nanoparticle for targeted delivery of Sorafenib tosylate towards the treatment of diabetic retinopathy. Colloids Surf. B Biointerfaces 2020, 194, 111151.
- Amato, R.; Giannaccini, M.; Dal Monte, M.; Cammalleri, M.; Pini, A.; Raffa, V.; Lulli, M.; Casini, G. Association of the Somatostatin Analog Octreotide with Magnetic Nanoparticles for Intraocular Delivery: A Possible Approach for the Treatment of Diabetic Retinopathy. Front. Bioeng. Biotechnol. 2020, 8, 144.
- Venkatesan, A.; Roy, A.; Kulandaivel, S.; Natesan, V.; Kim, S.-J. p-Coumaric Acid Nanoparticles Ameliorate Diabetic Nephropathy via Regulating mRNA Expression of KIM-1 and GLUT-2 in Streptozotocin-Induced Diabetic Rats. Metabolites 2022, 12, 1166.
- Huang, D.; Chen, Y.-S.; Thakur, S.S.; Rupenthal, I.D. Ultrasound-mediated nanoparticle delivery across ex vivo bovine retina after intravitreal injection. Eur. J. Pharm. Biopharm. 2017, 119, 125–136.
- Rassu, G.; Pavan, B.; Mandracchia, D.; Tripodo, G.; Botti, G.; Dalpiaz, A.; Gavini, E.; Giunchedi, P. Polymeric nanomicelles based on inulin D α-tocopherol succinate for the treatment of diabetic retinopathy. J. Drug Deliv. Sci. Technol. 2021, 61, 102286.
- Bogdanov, P.; Sampedro, J.; Solà-Adell, C.; Simó-Servat, O.; Russo, C.; Varela-Sende, L.; Simó, R.; Hernández, C. Effects of Liposomal Formulation of Citicoline in Experimental Diabetes-Induced Retinal Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 2458.
This entry is offline, you can click here to edit this entry!
