Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
A natural ecosystem can become contaminated due to the excessive release of toxic substances by various anthropogenic and natural activities, which necessitates rehabilitation of the environmental contamination. Phytoremediation is an eco-friendly and cost-efficient method of biotechnological mitigation for the remediation of polluted ecosystems and revegetation of contaminated sites.
- ecosystem
- degradation
- contamination
- restoration
- concentration
1. Phytoremediation
The term “phytoremediation” is composed of two roots: (i) the word “phyto” originates from Greek and connotes plant species; and (ii) the word “remedium” is Latin and means ability to treat or re-establish [1]. The technique implies using plant species to extract and eradicate elemental contaminants or reduce their biological availability in soil and water bodies and, hence, improve degraded environments [2]. In other words, it is an approach to lower the amount of contamination in polluted sites to environmentally tolerable levels by using green plants [3]. Phytoremediation consists of two systems: the root system hosting the microbial population and the plant itself, which transforms the toxic compounds to further non-toxic substances. It is an eco-friendly method for the rehabilitation of polluted ecosystems using plants and is relatively less expensive, as it is carried out in situ and exploits solar energy [4]. Plant species selection is a vital factor for successful phytoremediation and plants must possess the characteristics shown [5] (Figure 1). Fast-growing woody plant species, such as Populus and Salix, are easy to regenerate with extensive roots and have high evapotranspiration rates that stabilize pollutants; hence, they can flourish on soils with contaminated conditions. Such plants also have higher rates of biomass production and can resist a broad range of potentially harmful elements in their root systems [6]. The most important plant species may commonly be situated in colonizing contaminated places [7], such as plants emerging in mining sites. However, the most appropriate selection of plants for phyto-technology predominantly relies on the contaminant characteristics and their concentration levels [8], depth and volatilization rates [9][10], the age of the location [11] and biological degradability [12]. Furthermore, a clear knowledge of the water-use relations for a selected plant is also essential for the success of the phytoremediation approach.
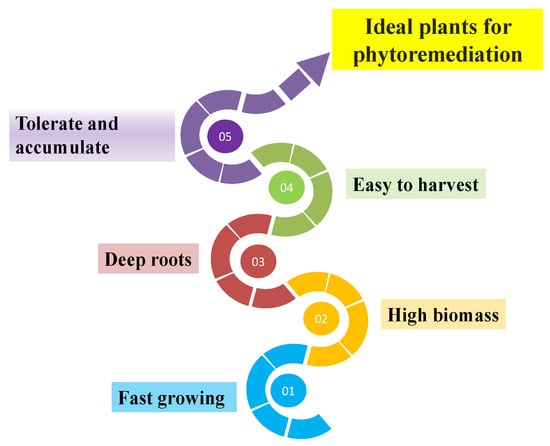
Figure 1. Characteristics of the ideal plants for phytoremediation.
2. Mechanism of the Phytoremediation Technique
On the basis of the type of contaminant, the media in which the treatment take place and the scope of application, the phyto-technique can be categorized into specific mechanisms (Table 1): phytoaccumulation/phytoextraction, rhizodegradation, phytostabilization, rhizofiltration, phytoevaporation/phytovolatilization, phytodesalination, phytodegradation/phytotransformation and phytohydraulics [13]. Once a plant takes up contaminants, it implements different detoxification mechanisms, such as accumulation, translocation, degradation, excretion or volatilization. The process of degradation involves transformation, fragmentation and/or deposition of the pollutant inside plant tissues [14].
Table 1. Various types of phytoremediation methods [15].
| Phytoremediation Type | Contaminant Nature | Medium | Mechanism | Scope of Application |
|---|---|---|---|---|
| Phytoextraction/phytoaccumulation | Inorganics | Soil, water | Hyperaccumulation | Moderately polluted sites |
| Rhizodegradation/phytostimulation | Organics | Soil | Breakdown inside the rhizosphere through microbial activity | Polycyclic aromatic hydrocarbon (PAH) contaminants |
| Phytostabilization | Inorganics | Soil | Immobilization | Mining contamination |
| Phytovolatilization/phytoevaporation | Organics/inorganics | Soil, water | Volatilization | Volatile contaminants |
| Rhizofiltration | Organics/inorganics | Water | Rhizosphere accumulation | Wastewater |
| Phytodegradation/phytotransformation | Complex organics | Water, soil | Breakdown inside the plant through metabolic processes | Soil and wastewater contamination |
| Phytodesalination | Organics/inorganics | Soil, water | Na hyperaccumulation | Sodic soil and water |
| Phytohydraulics | Organics/inorganics | Ground water | Uptake, sequestering and degradation of groundwater contaminants | Shallow contaminated sites |
2.1. Phytoextraction
Metal contamination impairs biological systems through direct consumption of metal-infected food, drinking contaminated water or the food chain as it can predispose living beings to serious health problems [16]. This type of contaminant does not undergo a process of biological degradation and, hence, necessitates physically eradication or transformation into non-toxic substances [17]. Phytoextraction/phytoaccumulation is the technique through which plants take up pollutants through their roots with subsequent translocation and accretion in their aboveground parts, which is generally followed by harvesting and final disposal of the biomass of the plant (Figure 2). Phytoextraction is also called phytosequestration and phytoabsorption. The process applies to metals (Cd, Ag, Cu, Cr, Co, Mo, Hg, Pb, Mn, Ni and Zn), radionuclides (137Cs, 90Sr, 238U and 234U), metalloids (Sb and As), non-metals (Se) and some organic substances (mainly hydrocarbons) since these do not usually have their structures further transformed or changed inside the plant system [18]. The occurrence of these contaminants in soil and water is the consequence of either natural forces, such as rock disaggregation, volcanoes and soil erosion, or human actions, such as incomplete fossil combustion, refining of metals, mineral mining, solid waste dumping, electronic product manufacturing, agricultural chemicals, pigments, vehicular gas emissions, military functions, etc. [15][19]. It is critical to conduct remediation operations to stop these heavy metals from penetrating terrestrial, aquatic and atmospheric environments, in addition to restoring the polluted ecosystem [20]. Therefore, phytoextraction is the technology through which plants extract heavy elements from water and soil media by creating complexes via chelation with elements/metals and their metabolites [21], thereby reducing their toxicity.
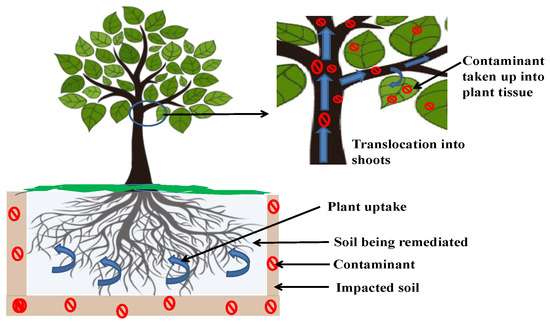
Figure 2. Mechanism of phytoextraction.
There are several factors responsible for the absorption mechanism of heavy metals, as discussed in the sections below.
2.1.1. Plant Species
The uptake of heavy metals is influenced by the nature of plant species. The special plants used for phytoextraction, named metallophytes, are divided into three categories, as follows [18]:
Metal excluders: These are the plants that prohibit metals from penetrating aerial shoots or retain low and stable levels of metal concentration across a wide variety of metal concentration levels in the soil. Some examples of excluder species include Oenothera biennis, Commelina communis, Salix spp., Silene maritime and Populus spp. [22];
Metal indicators: An indicator is defined as a plant species containing the same levels of heavy metals in its tissues as in the surrounding soil environment [23]. They can tolerate the present concentration level of metals by forming chelators (intracellular element-binding substances) or by sequestering metals within the plant biomass [10]. Such plants are of bio-ecological significance because they are indicators of pollution and also resemble accumulators in the way they take up pollutants. Examples are Pluchea dioscoridis, Agave sisalana and Cyperus articulates [24];
Hyperaccumulators: Hyperaccumulators are plants that contain higher levels of metal pollutants concentrated in their root systems, shoots or foliage [25]. The variation in the absorbing potential of different hyperaccumulators relies on the occurrence, regulation and expression of responsible genes and the adjoining environment [26]. Metal-accumulating plants can concentrate most heavy metals, such as Co, Cd, Zn, Ni, Pb and Mn, at levels equal to 100 or 1000 times those in excluder plant species [17]. To date, over 500 plants from above 40 genera have been recognized as natural hyperaccumulating species, comprising <0.2% of the total angiosperms [27]. Woody plant species, such as poplars and willows, are utilized for phytoextraction because of several advantages, as they have high biomass in comparison to shrubs and herbs, which promotes the absorption of high concentrations of metals in their aboveground shoots. Furthermore, they possess a deep root system, which lessens soil erosion and helps to avoid the movement of polluted soil to adjoining locations [20].
2.1.2. The Properties of the Medium
The remediation treatment for a contaminated ecosystem can be boosted by employing agronomical practices, such as fertilizers, chelator addition and adjustment of pH.
2.1.3. Root Zone
Plant roots can accumulate pollutants and sequester or metabolize them within plant parts. Roots release root exudates, which cause degradation of pollutants in the growing soil media.
2.1.4. Environmental Conditions
Environmental conditions affect the uptake mechanism. For instance, temperatures affect growth materials and, subsequently, the root length of the plant.
2.1.5. Chemical Properties of Contaminants
Phytoextraction relies on contaminant-specific hyperaccumulators and the consequential metabolic products of the contaminants within plants.
2.1.6. Bioavailability of Metals
The biological availability of metals in the soil is defined as the portion of the total metals in the interstitial space of the water and soil particles accessible to the receptor organisms [28]. Metal accumulation by plants is reliant on the bioavailability of metals, which in turn depends on the upholding capacity time of the metals, as well as their interaction with other compounds in the growing media. Plants affect the soil by enhancing the biological availability of metals by adding physicochemical and biodegradable factors.
2.1.7. Chelating Agent Addition
The uptake of heavy metals can be improved by adding biodegradable substances, such as micronutrients and chelating agents. Moreover, in the process of chelate-assisted remediation, chemical chelating agents, such as ethylenediaminetetraacetic acid (EDTA) and nitrilotriacetic acid (NTA), are supplied to improve the phytoextraction of soil-contaminating metals.
2.2. Rhizodegradation
Rhizoremediation is the degradation of different organic contaminants in growing soil through microbial activity, and it can be enhanced by the existing root zone. It is also called phytostimulation, plant-assisted remediation and rhizosphere biodegradation [29] (Figure 3a). Rhizodegradation is recognized as the most efficient method for polycyclic aromatic hydrocarbon (PAH) treatment in soil. The combined action of the microbial population and plant root system in the rhizosphere, such as the discharge of root exudates (amino acids, sugars, organic acids, etc.), hydrogen cyanide (HCN), siderophores and phosphatases and phytohormone production by plant growth-promoting rhizobacteria (PGPR), helps in the breakdown of contaminants [30]. PAHs originate from either natural or anthropogenic activities; among them, the combustion of organic matter is the major source of contamination (Figure 3b). The mechanism of rhizodegradation involves direct phytohormonal activity, enhancing nutrient availability and regulating microbial population, which makes it possible to degrade contaminants by using a growing root system [31]. The pollutants taken up by plants are either accumulated in harvestable parts or volatilized through leaves or stems (Figure 3c). Plant–microbe interactions, soil parameters and the conditions that promote degradation rates are the factors affecting the rate of rhizodegradation (Figure 3d). Soil characteristics limit the bioavailability of contaminants, and root–microbe associations are the key process in PAH phytoremediation.
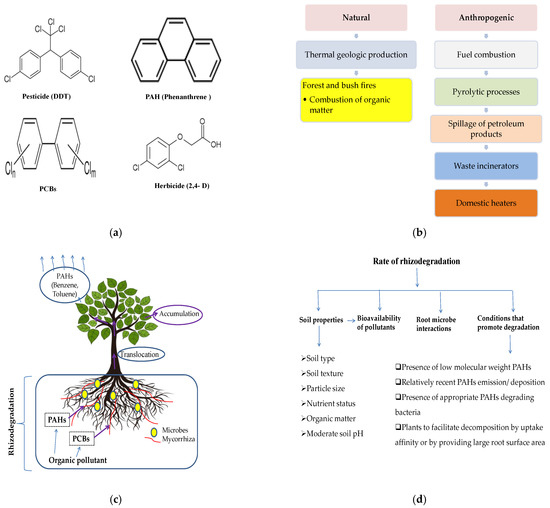
Figure 3. Rhizodegradation techniques: (a) chemical structures of different organic contaminants, (b) sources of PAH contamination, (c) mechanisms of rhizoremediation, and (d) factors affecting the rate of rhizodegradation.
2.3. Phytostabilization
Phytostabilization, also known as phytoimmobilization, is the process of making a contaminant immobile (immovable) in soil media via (a) absorption by the plant roots, (b) adsorption on the roots or (c) precipitation in between the rhizospheres of growing media (Figure 4a). The technique reduces the mobility of contaminants by stabilizing them via the development of a vegetative cover at the root zone of the plant species, helping to avoid migration to the groundwater. Microbial growth correlated with the plant root system may enhance the breakdown of organic contaminants, such as petroleum hydrocarbons and pesticides, to likely non-toxic compounds [28]. Plant, soil, contaminant and environmental factors determine the success of phytostabilization technology in contaminated sites in terms of both revegetation and restoration (Figure 4b). Phytostabilization can be enhanced by increasing biological inoculants, such as plant growth-promoting bacteria (PGPB), organic amendments (biosolids, manures) and inorganic amendments (liming materials, clay materials and phosphate compounds), and through geotextile capping.
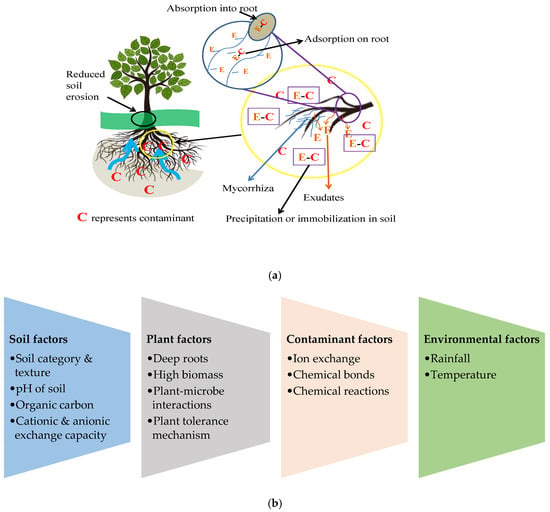
Figure 4. (a) Mechanisms of phytostabilization and (b) factors affecting the process of phytostabilization.
2.4. Phytovolatilization
Phytovolatilization is the absorption, translocation and evaporation of a pollutant by a plant species, with a modified type of pollutant being discharged into the adjoining atmosphere. Phytoevaporation or phytovolatilization occurs when growing trees and other plant species consume water and pollutants simultaneously, resulting in volatilization through foliage or stems (direct phytovolatilization) or the soil surface because of the root activities of the plant (indirect phytovolatilization process) (Figure 5a). The phytoevaporation process can be employed for detoxification of both hazardous inorganic and organic contaminants [20]. Volatile types of hazardous inorganic substances, such as As, Se and Hg, can be evaporated from plant parts [14].
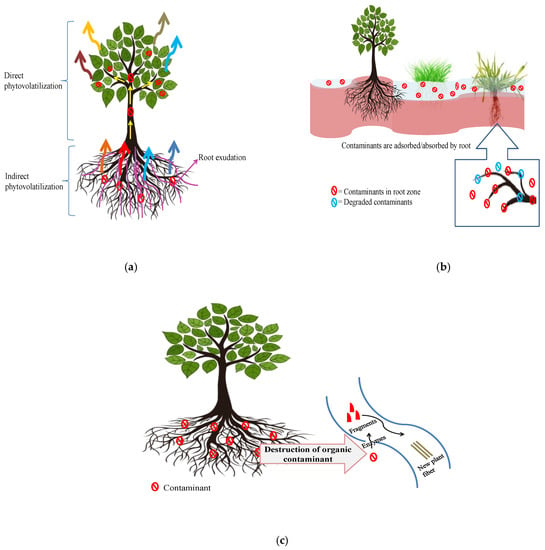
Figure 5. Mechanisms of (a) phytovolatilization, (b) rhizofiltration, and (c) phytodegradation.
2.5. Rhizofiltration
Rhizofiltration is a technique for the assimilation of pollutants present in a solution adjoining the root zone, concentrating and precipitating them on or into plant roots through biotic or abiotic methods (Figure 5b). This approach is mostly employed to rehabilitate polluted groundwater sources. Plant roots release root exudates (secondary metabolites) that undergo various biogeochemical processes, resulting in the precipitation of pollutants onto roots or into water bodies. Later on, adsorption of contaminants onto or into the plant root system and translocation to the aboveground portions occur based on the type of plant species and the pollutant type and concentration [32]. Plant species, the nutrient status and chemical characteristics of contaminants and the conditions of the groundwater are the prime factors that are used to evaluate the effectiveness of the rhizofiltration method in treating inorganic (metals) and organic pollutants present in groundwater resources.
2.6. Phytodegradation
The phytodegradation process involves the transformation of contaminants absorbed by plants through metabolic pathways inside the plant system (Figure 5c). It is also known as phytotransformation. The complex organic contaminants are converted into simpler products and integrated into the plant tissues to boost plant growth [33]. Plants release specific enzymes that catalyze and enhance the rate of transformation. The phytodegradation technique can be used for the treatment of chemical pollutants; for instance, chlorinated solvents, herbicides and explosives in groundwater sources, aromatic and petroleum hydrocarbons in growing soils and volatile substances from the atmosphere [34]. In environmental applications, this process relies upon the direct absorption of contaminants from soil water and the resultant metabolites accumulated within the plant tissues, provided that the accumulated metabolites are either non-toxic or less toxic than the original contaminant.
2.7. Phytodesalination
Phytodesalination is an eco-friendly technology that is employed to remediate water resources and sodic soils by using Na-hyperaccumulating halophytes. It is the process through which plant species are employed as desalination agents [35], which means the use of the halophytes’ ability to absorb huge quantities of sodium (Na+) ions from affected ecosystems and their elimination by means of accumulation and translocation to different harvestable plant parts (Figure 6a) [36]. Saline-sodic soils cover almost 6% (over 800 million ha) of the land surface worldwide and, to overcome this problem, several authors have emphasized the phytodesalination technique by proving the effectiveness of Na+-hyperaccumulating plants in desalinizing the soil, especially in arid and semi-arid locations [37]. This is because halophytes decrease the ion concentration level of the solution in the plant xylem and cause Na+ and Cl− excretion at elevated levels of salt concentration [38]. The potential of phytodesalination is species-specific (Figure 6b) and dependent on soil and climatic conditions (primarily rainfall).
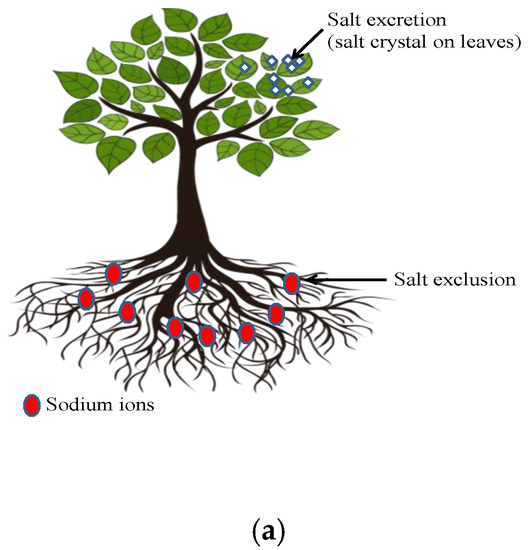
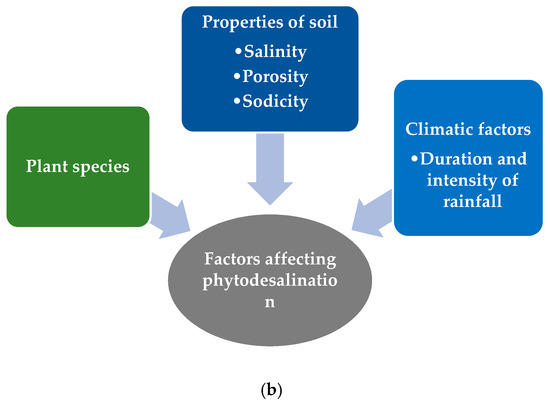
Figure 6. Phytodesalination process: (a) mechanism of phytodesalination and (b) factors affecting the mechanism of phytodesalination.
2.8. Phytohydraulics
Phytohydraulics is the exploitation of deep-rooted plant species to uptake, sequester and breakdown groundwater pollutants that emerge in contact with their root systems (Figure 7). A special class of plant species called phreatophytes are widely used for this purpose. Phreatophytes are deep-rooted, water-loving plants that have high transpiration rates and penetrate their roots into zones of high moisture, and they can also continue to exist under temporary saturation conditions [39]. Prosopis, Eucalyptus, Populus and Salix are typical phreatophytes.
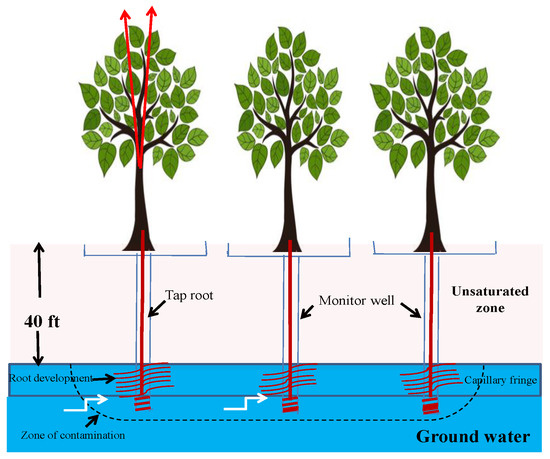
Figure 7. Mechanism of phytohydraulics.
This entry is adapted from the peer-reviewed paper 10.3390/w15081498
References
- Vamerali, T.; Bandiera, M.; Mosca, G. Field crops for phytoremediation of metal-contaminated land. A review. Environ. Chem. Lett. 2010, 8, 1–17.
- Robinson, B.H.; Lombi, E.; Zhao, F.J.; Mcgrath, S.P. Uptake and distribution of nickel and other metals in the hyperaccumulator Berkheya Coddii. New Phytol. 2003, 158, 279–285.
- Borisev, M.; Pajevic, S.; Nikolic, N.; Pilipovic, A.; Krstic, B.; Orlovic, S. Phytoextraction of Cd, Ni, and Pb Using Four Willow Clones (Salix spp.). Pol. J. Environ. Stud. 2009, 18, 553–561.
- Tripathi, V.; Edrisi, S.A.; Abhilash, P.C. Towards the coupling of phytoremediation with bioenergy production. Renew. Sustain. Energy Rev. 2016, 57, 1386–1389.
- Bokhari, S.H.; Ahmad, I.; Mahmood-Ul-Hassan, M.; Mohammad, A. Phytoremediation potential of Lemna minor L. for heavy metals. Int. J. Phytoremediat. 2016, 18, 25–32.
- Wani, K.A.; Sofi, Z.M.; Malik, J.A.; Wani, J.A. Phytoremediation of Heavy Metals Using Salix (Willows). In Bioremediation and Biotechnology; Bhat, R., Hakeem, K., Dervash, M., Eds.; Springer: Cham, Switzerland, 2020; p. 2.
- Farraji, H.; Zaman, N.Q.; Tajuddin, R.; Faraji, H. Advantages and disadvantages of phytoremediation: A concise review. Int. J. Environ. Sci. Technol. (IJEST) 2016, 2, 69–75.
- Pilon-Smits, E. Phytoremediation. Annu. Reviewof. Plant Biol. 2005, 56, 15–39.
- Cunningham, S.D.; Anderson, T.A.; Schwab, A.P.; Hsu, F.C. Phytoremediation of Soils Contaminated with Organic Pollutants. In Advances in Agronomy; Academic Press Inc.: Cambridge, MA, USA, 1996; Volume 56, pp. 55–114.
- Ghosh, M.; Singh, S.P. A Review on Phytoremediation of Heavy Metals and Utilization of Its Byproducts. Appl. Ecol. Environ. Res. 2005, 3, 1–18.
- Hutchinson, S.L.; Banks, M.K.; Schwab, A.P. Phytoremediation of aged petroleum sludge: Effect of inorganic fertilizer. J. Environ. Qual. 2001, 30, 395–403.
- Joner, E.; Leyval, C. Phytoremediation of organic pollutants using mycorrhizal plants: A new aspect of rhizosphere interactions. Agronomie 2003, 23, 495–502.
- Awa, S.H.; Hadibarata, T. Removal of Heavy Metals in Contaminated Soil by Phytoremediation Mechanism: A review. Water Air Soil Pollut. 2020, 231, 47.
- Limmer, M.; Burken, J. Phytovolatilization of Organic Contaminants. Environ. Sci. Technol. 2016, 50, 6632–6643.
- Bhat, S.A.; Bashir, O.; Haq, S.A.U.; Amin, T.; Rafiq, A.; Ali, M.; Americo-Pinheiro, J.H.P.; Sher, F. Phytoremediation of heavy metals in soil and water: An eco-friendly, sustainable and multidisciplinary approach. Chemosphere 2022, 303, 100774.
- Kumar, A.; Kumar, A.; Mms, C.-P.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179.
- Tangahu, B.V.; Abdullah, S.R.S.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161.
- Babu, S.M.O.F.; Hossain, M.B.; Rahman, M.S.; Rahman, M.; Ahmed, A.S.S.; Hasan, M.M.; Rakib, A.; Emran, T.B.; Xiao, J.; Simal-Gandara, J. Phytoremediation of Toxic Metals: A Sustainable Green Solution for Clean Environment. Appl. Sci. 2021, 11, 10348.
- Mandal, A.; Purakayastha, T.J.; Ramana, S.; Neenu, S.; Bhaduri, D.; Chakraborty, K.; Manna, M.C.; Rao, A.S. Status on Phytoremediation of Heavy Metals in India—A Review. Int. J. Bio-Resour. Stress Manag. 2014, 5, 553–560.
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd –Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359.
- Yaqoob, A.; Nasim, F.H.; Sumreen, A.; Munawar, N.; Zia, M.A.; Choudhary, M.S.; Ashraf, M. Current scenario of phytoremediation: Progresses and limitations. Int. J. Biosci. 2019, 14, 191–206.
- Mehes-Smith, M.; Nkongolo, K.; Cholew, E. Coping Mechanisms of Plants to Metal Contaminated Soil. In Environmental Change and Sustainability; Silvern, S., Young, S., Eds.; Intech Open: London, UK, 2013.
- Baker, A.J.M.; Walker, P.L. Ecophysiology of metal uptake by tolerant plants: Heavy metal tolerance in plants. In Evolutionary Aspects; Shaw, A.J., Ed.; CRC Press: Boca Raton, FL, USA, 1990.
- Mganga, N.; Manoko, M.L.K.; Rulangaranga, Z.K. Classification of Plants According to Their Heavy Metal Content around North Mara Gold Mine, Tanzania: Implication for Phytoremediation. Tanzan. J. Sci. 2011, 37, 109–119.
- Cunningham, S.D.; Ow, D.W. Promises and Prospects of Phytoremediation. Plant Physiol. 1996, 110, 715–719.
- Rascio, N.; Navari-Izzo, F. Heavy Metal Hyperaccumulating Plants: How and Why Do They Do It? And What Makes Them So Interesting? Plant Sci. 2011, 180, 169–181.
- Bian, X.; Cui, J.; Tang, B.; Yang, L. Chelant-Induced Phytoextraction of Heavy Metals from Contaminated Soils: A Review. Pol. J. Environ. Stud. 2018, 27, 2417–2424.
- Bolan, N.S.; Park, J.H.; Robinson, B.; Naidu, R.; Huh, K.Y. Phytostabilization: A Green Approach to Contaminant Containment. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 112, pp. 145–204.
- Kumar, A.; Mishra, S.; Pandey, R.; Yu, Z.G.; Kumar, M.; Khoo, K.S.; Thakur, T.K.; Show, P.L. Microplastics in terrestrial ecosystems: Un-ignorable impacts on soil characterises, nutrient storage and its cycling. TrAC Trends Anal. Chem. 2023, 158, 116869.
- Shukla, K.P.; Sharma, S.; Kumar, N.; Singh, V.; Bisht, S.; Kumar, V. Rhizoremediation: A Promising Rhizosphere Technology. In Applied Bioremediation—Active and Passive Approaches; Patil, Y.B., Rao, P., Eds.; IntechOpen Limited: London, UK, 2013.
- Lee, S.; Ka, J.O.; Gyu, S.H. Growth promotion of Xanthium italicum by application of rhizobacterial isolates of Bacillus aryabhattai in microcosm soil. J. Microbiol. 2012, 50, 45–49.
- Kristanti, R.A.; Ngu, W.J.; Yuniarto, A.; Hadibarata, T. Rhizofiltration for Removal of Inorganic and Organic Pollutants in Groundwater: A Review. Biointerface Res. Appl. Chem. 2021, 11, 12326–12347.
- Greipsson, S. Phytoremediation. Nat. Educ. Knowl. 2011, 3, 7.
- Newman, L.A.; Reynolds, C.M. Phytodegradation of organic compounds. Curr. Opin. Biotechnol. 2004, 15, 225–230.
- Sarath, N.G.; Sruthi, P.; Shackira, A.M.; Puthur, J.T. Halophytes as effective tool for phytodesalination and land reclamation. In Frontiers in Plant-Soil Interaction; Academic Press: Cambridge, MA, USA, 2021; pp. 459–494.
- Lastiri-Hernandez, M.A.; Alvarez-Bernal, D.; Bermudez-Torres, K.; Cardenas, G.C.; Ceja-Torres, L.F. Phytodesalination of a moderately saline soil combined with two inorganic amendments. Bragantia 2019, 78, 579–586.
- Zorrig, W.; Rabhi, M.; Ferchichi, S.; Smaouti, A.; Abdelly, C. Phytodesalination: A Solution for Salt-affected Soils in Arid and Semi-arid Regions. J. Arid. Land Stud. 2012, 22, 299–302.
- Saddhe, A.A.; Manuka, R.; Nikalje, G.C.; Penna, S. Halophytes as a Potential Resource for Phytodesalination. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020.
- Gatliff, E.G. Vegetative Remediation Process Offers Advantages over Traditional Pump and-Treat Technologies. Remediation 1994, 4, 343–352.
This entry is offline, you can click here to edit this entry!
