Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The intestinal immune balance is disrupted by a high-fat diet (HFD) in several ways, such as impairing the intestinal barrier, influencing immune cells, and altering the gut microbiota. In contrast, a rational diet is thought to maintain intestinal immunity by regulating gut microbiota.
- high-fat diet
- inflammatory bowel disease
- intestinal barrier
- intestinal microflora
1. Introduction
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), causes significant gastrointestinal symptoms, such as diarrhea, abdominal pain, bleeding, and weight loss. IBD is associated with a spectrum of extraintestinal manifestations, including sclerosing cholangitis, uveitis, arthritis, erythema nodosum, and pyoderma gangrenosum [1]. The steadily increasing emergence of IBD has cause great burden to the health care system and society worldwide; therefore, it is important to explore its underlying pathogenesis. Although identifying its exact pathogenesis remains difficult, overwhelming data suggests that its prevalence is related to Western diets [2] which feature high saturated fat, high sugar (HS), and low fiber exposure [3][4]. Similar to Western diets, a high fat diet (HFD) contains high amounts of fatty acids and is low in fiber and vitamins [5]. This dietary shift has been proposed as an etiological factor of IBD because the accelerating incidence of IBD in newly industrialized countries is in line with the westernized dietary structure [6][7][8]. The severity of IBD is also influenced by hormonal regulation under food intake [9]. For example, ghrelin is not only a multi-faceted gut hormone that regulates gastrointestinal motility, stimulates food intake, and promotes fat deposition [10]; it is also a mediator to prevent intestinal inflammation [10][11]. Research demonstrates that ghrelin supplements can alleviate inflammation in different models of IBD or accelerate the healing of gastrointestinal ulceration [12][13][14][15][16][17], while consequently a high fat diet could decrease levels of ghrelin and impair its anti-inflammatory effect [18]. Additionally, HFD can reduce the secretion of somatostatin and increase the level of serotonin [19][20], resulting in the exacerbated inflammation of IBD [20][21]. Therefore, to adopt appropriate therapies to treat IBD, it is imperative to uncover the mechanisms between diet and IBD and their potential implication in reducing IBD morbidity, achieving IBD remission, and reducing the disease burden on patients and healthcare systems.
Intestinal immunity, as a pivotal factor in IBD pathogenesis, cannot be underestimated in IBD progression because of its unique roles in warding off pathogen invasion from the gut and retaining host–microbial immune homeostasis [22]. The dysfunction of intestinal immunity may lead, for various reasons, to increased intestinal permeability and makes it easier for pathogens and their products to enter host tissues, thus resulting in worsening conditions [23]. Therefore, increased intestinal permeability has significance in predicting disease recurrence in IBD [24][25]. Moreover, the dysregulation of adaptive immunity is also a predictor of the clinical course of IBD [26], and genetics are also essential participants in these immune processes [27]. These studies conclude that intestinal immunity is intimately involved in the pathogenesis of IBD. The intestinal microbiota has been linked to the development of IBD for many years. Firmicutes and Bacteroidetes are the predominant bacteria in the human intestine; however, a lower proportion of these species was found in IBD patients, both in children and adults [28]. Notably, these two obligate anaerobes are responsible for catabolizing fiber into short-chain fatty acids. A reduction in their abundance would reduce the production of short chain fatty acids and result in IBD occurrence or accelerate the IBD course due to the loss of their anti-inflammatory properties [29][30].
Additionally, an increase in adherent–invasive E. Coli (AIEC), which plays a pathogenic effect on disease development with the ability to adhere and invade intestinal epithelial cells [31], was also displayed in CD patients. The role of gut microbiota composition and function in the development and severity of IBD is demonstrated by the changes in microbiota composition among IBD patients observed in these studies. Engineering the microbiota as an adjunct to boost current IBD therapies would be a promising approach.
Because of the meaningful roles of diet, intestinal immunity, and microbiota in IBD, many studies have attempted to explore their links to the three entities in IBD (Figure 1).
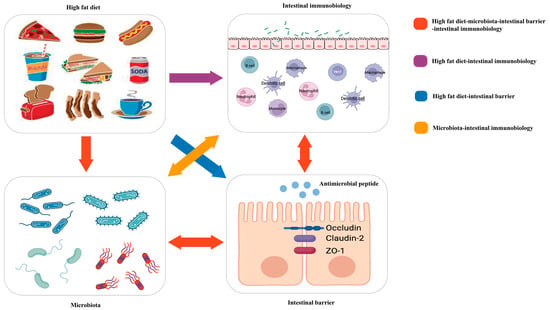
Figure 1. Red arrow: the high-fat diet shapes the gut microbiota, which changes the function of the intestinal barrier and further influences intestinal immunobiology. The intestinal immunobiology also affects the intestinal barrier and IECs, i.e., the components of the intestinal barrier, which also influence microbiota. Purple arrow: the high-fat diet modulates immune cell infiltration and polarization. Blue arrow: the high-fat diet influences the intestinal barrier. Orange arrow: gut microbiota interacts with intestinal immunobiology.
Researchers will focus on the critical roles of a high-fat diet (HFD) on intestinal immunity and gut microbiota and the impacts of the altered intestinal microecological imbalance on intestinal immunity in IBD, aiming to offer a direction for the pathogenesis of IBD (Figure 2).
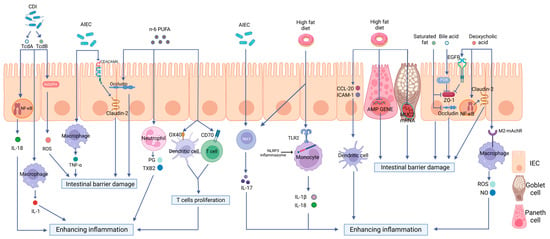
Figure 2. The effects of a high-fat diet on intestinal immunity in IBD. The specific manifestations are as follows: (1) Intestinal barrier dysfunction. A high-fat diet (HFD) disrupts the intestinal tight junction (TJ) structure and reduces Mucin 2 abundance and antimicrobial peptide (AMP) secretion. (2) Dysregulated PRR activation. HFD activates Toll-like receptor 2(TLR2) and consequently activates the NLRP3 inflammasome and promotes the release of inflammatory mediators, such as interleukin-18 (IL-18) and interleukin-1 beta (IL-1β), through monocytes to enhance intestinal inflammation. (3) The disordered function of immune cells. HFD can increase the production of OX40 and CD70 in both DCs and T cells, therefore inducing T cell proliferation and causing a proinflammatory effect. HFD can also stimulate the maturation of DCs, the polarization of Th17 cells, neutrophil migration, and the release of inflammatory mediators. (4) Intestinal dysbiosis. HFD can increase the abundance rates of AIEC and Clostridioides difficile, causing intestinal barrier disruption and enhancing intestinal inflammation via multiple mechanisms.
2. High-Fat Diet and the Intestinal Barrier in IBD
Intestinal epithelial cells (IECs) and tight junctions (TJs) constitute the epithelial barrier of intestinal immunity; its integrity is the precondition for intestinal defense [32]. Mucosal defense, as the front of the epithelial barrier, including immune molecules and mucus proteins, represents the most prominent interaction zone between the microbiota and host [33]. The receptors expressed in the intestinal epithelial cells also play vital roles in the constant communication of gut microflora and immune cells aggregated in the mucosal epithelium or submucosal lamina propria. Various studies have shown that an abnormal intestinal barrier function is crucial to the pathogenesis of IBD [34][35][36]. Moreover, HFD was shown to participate in intestinal inflammatory responses by affecting the intestinal barrier function [37] (Figure 2).
3. High-Fat Diet and Pattern Recognition Receptors in IBD
Pattern recognition receptors (PRRs) are immune sensors that recognize pathogen-associated molecular patterns (PAMPs) inside and outside the cells. Many PRRs, such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs) and the molecules interacting with them, have been identified and characterized. PRRs are essential in the detection and elimination of pathogens. However, dysregulated PRR activation can lead to inflammatory diseases. It has been shown by increasing evidence that innate immune dysfunction, which is mediated by TLRs and NLRP3, plays a crucial role in the pathogenesis of IBD [38][39]. Previous research has shown that PRR-mediated inflammation, which is caused by an HFD, plays a notable role in intestinal inflammation [40][41][42][43][44][45] (Figure 3).
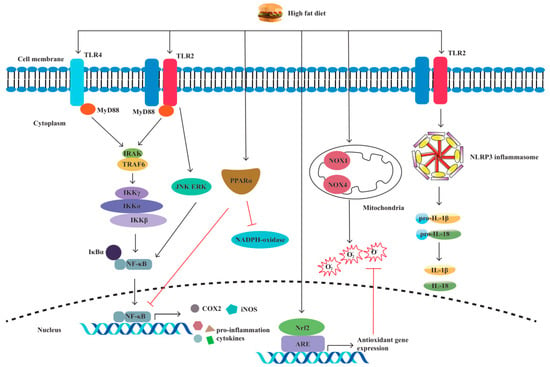
Figure 3. A high-fat diet regulates proinflammatory factors and oxidative stress through TLRs and NLRP. The specific manifestations are as follows: (1) Through the TLR4/NF−κB/MyD88 pathway, a high-fat diet (HFD) causes intestinal inflammation by elevating cytokine levels and alleviating cellular oxidative stress. (2) HFD activates oxidative stress markers nicotinamide adenine dinucleotide phosphate (NADPH), NADPH oxidase 4 (NOX4), and NOX1. (3) HFD increases the production of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). (4) HFD activates TLR2, resulting in M1 macrophage polarization and proinflammatory cytokine production. (5) HFD induces NLRP3 expression and promotes pro-IL-1β and IL-1β secretion by activating TLR2.
4. High-Fat Diet and Immune Cells in IBD
The disordered function and the number of adaptive immune cells, especially T lymphocytes, are crucial to IBD etiology [46][47][48][49] (Figure 2). A regulatory role of HFD in adaptive immune cells was also demonstrated in HFD-fed DSS-induced colitis mice; increased non-CD1d-restricted NKT cells and decreased Tregs were observed in the colon. This is paralleled by the fact that a higher ratio of effector T cells/regulatory T cells will contribute to the inflammatory state in the intestine [50]. Moreover, HFD can disrupt the steady state of intraepithelial lymphocytes (IELs), reducing homeostatic proliferation in intraepithelial T lymphocytes and the expression of CD103 and CCR9 on these cells to worsen the outcome of DSS-induced colitis in mice [51]. Similarly, HFD-fed mice substantially increased Th17 polarization [52] and CD3+ T cell infiltration in the gut [53], resulting in colitis aggravation. HFD-produced peroxidized lipids, such as 13-HPODE, could stimulate the secretion of pro-inflammatory granzymes by resident NK cells, thereby contributing to intestinal inflammation [54]. Previous studies of IBD suggest the involvement of innate lymphoid cells (ILCs) [55]. There was an increase in IL-17-producing type 3 innate lymphoid cells (ILC3s) in the offspring of mice that were fed a high-fat diet, leading to high susceptibility to inflammation [56]. In another animal study, significantly increased expression of caspase-3 was found in type 1 innate lymphoid cells (ILC1) and ILC3 in the mice fed with HFD as compared to the control group, possibly resulting in pro-apoptotic mechanisms [57]. In terms of B cells, in the plasma and spleen of C57BL/6 mice, the HFD induced evident decreases in the number of B cells, accompanied by oxidative stress and increased oxidative damage [58]. Gurzell et al. [59] found that docosahexaenoic acid (DHA), a type of n-3 polyunsaturated fatty acid that is low in Western diets, enhances B cell activation, thereby boosting the humoral immunity of mice, which may up-regulate the resolution phase of inflammation.
Furthermore, an increased presence of colonic macrophages has been shown in HFD-induced obese mice [60]. Still, they are deficient in forkhead box O3 (FOXO3) in macrophages [60], which mediates the proapoptotic or anti-inflammatory effects of macrophages [61]. Neutrophils, generally regarded as keys to inflammation, play a crucial role in intestinal inflammation in IBD [62]. Neutrophil migration is enhanced by an HFD due to the elevated expression of associated cytokines [63], such as monocyte chemoattractant protein-1 (MCP-1) [64] and chemokine (C-X-C motif) ligands 1 (CXCL1) and 2 (CXCL2), in the intestine of mice [65]. Yoshida et al. [66] found that the secretion of growth-regulated oncogene/cytokine-induced neutrophil chemoattractant-1 (GRO/CINC-1) could be increased by long-chain fatty acids in rat IECs.
In a mice model of Crohn’s disease-like ileitis, DCs recruited into the intestinal lamina propria also appear because of the enhanced levels of chemokine (C-C motif) ligand (CCL) 20 and intercellular cell adhesion molecule 1 (ICAM1) induced by the HFD [67]. Moreover, increased maturity markers of DCs were found in a DSS-induced colitis model that was fed an HFD, which exacerbated intestinal inflammation [44][68].
All these data illustrate that an HFD could influence intestinal immune cells and the humoral immune response to induce intestinal inflammation, disrupt tissue structure, and exacerbate IBD conditions. More emphasis should be placed on the fact that some studies have concentrated on the changes in these cytokines and immune cells under an HFD but have not explored the alterations in intestinal microorganisms. Whether these immunity-related changes are just the results of this dietary model, the results of intestinal microorganisms, or both, is still obscure. Therefore, it is necessary to conduct these studies in germ-free mice, which may better elaborate on the direct effect of diet on intestinal immunity and help explain how environmental factors can increase susceptibility to IBD.
5. Polyunsaturated Fatty Acids (PUFAs) in IBD
Polyunsaturated fatty acids (PUFAs) are a type of fatty acid that contains more than two double bonds. PUFAs contain two principal families: n-6 (or omega-6) and n-3 (or omega-3). Eicosapentaenoic acid (EPA) and DHA are precursors of n-3 PUFAs and are classified as essential lipid mediators. The typical Western-style diet has a high n-6/n-3 ratio of approximately 10–15:1 [69]. The increase in dietary n-6/n-3 PUFAs was positively correlated with the increased incidence of IBD [70] (Figure 2). John et al. [71] suggested that increasing the consumption of n-3 PUFAs may help prevent UC. In addition, prospective research has revealed that increasing the proportion of n-3/n-6 PUFA ingestion can help to maintain IBD remission [72].
Beguin et al. [73] showed that 150 mM of DHA could increase ZO-1 intensity in vitro. A lower intensity of occludin, when incubated with n-6 PUFAs, was also found. Previous research has shown that TLR-2 gene expression in TNBS-induced colitis may be promoted by n-3 PUFAs [74]. The effect of n-3 PUFAs on neutrophils in the inflammatory process has also been investigated in TNBS mice in vivo; DHA and EPA could inhibit PMN transepithelial migration by reducing VCAM-1 and ICAM-1 [74]. Studies have revealed that the serum level of leukotriene B4 (LTB4) secreted by neutrophils in UC patients could be reduced by n-3 PUFAs and that the extravascular tissue damage caused by excessively activated neutrophils could also be avoided [75]. Current studies in mice show that n-3 polyunsaturated fatty acids could reduce the antigen-presenting function of DCs by inhibiting the expression of CD69 and CTLA-4 on T lymphocytes [76][77]. The metabolites of linoleic acid (LA, n-6 PUFAs) and arachidonic acid (AA, n-6) can produce thromboxane B2 (TXB2) and 4-series leukotrienes (LTS) through the cyclooxygenase (COX) pathway [78]. Additionally, n-6 PUFAs could also promote the production of PGE2 in vitro, thereby increasing the production of costimulatory molecules, including OX40 and CD70 in both DCs and T cells; this could induce T-cell proliferation and cause proinflammatory effects [79][80]. Moreover, the role of n-3 PUFAs in distinct types of enteritis models seems to differ. In the chronic model of intestinal inflammation, higher levels of suppressive cytokines are expressed by Th17 cells in the colon of mice than in the spleen. At the same time, there was no difference in the acute model [81][82]. Therefore, the relationship between PUFAs and T cells, especially Th17 cells in IBD, requires further study.
6. Short Chain Fatty Acids (SCFAs) in IBD
SCFAs are the products of anaerobic fermentation of dietary fiber, primarily containing acetate, propionate, and butyrate [8][83]. Nowadays, the beneficial roles of SCFAs on intestinal barrier integrity and immune cell functions in IBD have been highlighted by increasing evidence. Zheng et al. [84] revealed that claudin-2 formation is negatively regulated by butyrate via upregulating IL-10RA expression to protect gut barrier function, which was related to the signal transducing activator of transcription 3 (STAT3)-Histone Deacetylase inhibition (HDACi) pathway. Moreover, Hatayama et al. [85], who treated the human colon cancer cell line with butyrate and confirmed that SCFAs stimulates MUC2 production both in protein and mRNA levels, revealed that SCFAs increase MUC2 production.
The mechanism of butyrate stimulating AMP production has been investigated by Zhao et al. [86]. They used mammalian target of rapamycin (mTOR) siRNA and STAT3 siRNA to knockdown mTOR and STAT3, respectively, in intestinal epithelial cell (IEC) models and found that the mRNA and protein expressions of RegIIIγ and β-defensins were prominently impaired in these IECs, thus indicating that butyrate could active mTOR and STAT3 to promote AMP synthesis and confer resistance to colitis. The STAT3 and mTOR pathways have also been demonstrated to promote Th1 cells producing IL-10 by using butyrate to deal with the T cells from IBD patients and the DSS model, therefore limiting colitis [87]. Noteworthy, the role of SCFAs on T cells is related to the cytokine milieu. In the case of SCFA treatment, effector T cells, including Th1 and Th17 cells, are generated from naive T cells under a steady inflammatory condition, whereas in the active immune responses, the productions are regulatory T cells, such as IL-10+ T cells and FoxP3+ T cells [88]. Meanwhile, DC differentiation has also been reflected to be associated with mTOR and SATA3 pathways [89]. Butyrate, as a HDAC3 inhibitor, increases the antimicrobial functions of intestinal macrophages through a reduction in mTOR kinase activity [90] and downregulates macrophages secreting proinflammatory mediators [91]. The effect of SCFAs on neutrophils in the inflammatory process has also been investigated both in rats vivo and in vitro. On the one hand, SCFAs can promote neutrophils recruited into inflammatory sites by increasing L-selectin expression and chemokine release [92]. On the other hand, pro-inflammatory cytokines, TNF-α, and NO, produced by lipopolysaccharide (LPS)-stimulated neutrophils, are inhibited by SCFA treatment [93]. In view of the fact that mTOR is crucial in regulating the differentiation and function of innate and adaptive immune cells for intestinal immunity and that STAT3 has prominent aspects in the expressions of cytokines and chemokines, great insights must be explored about the interrelations between immune homeostasis and SCFAs in IBD.
7. High-Fat Diet and Intestinal Dysbacteriosis in IBD
It is well acknowledged that IBD is linked to compositional and metabolic alterations in intestinal microbiota. Fecal microbiota transplantation has been reported as a potential treatment of IBD [94][95]. Microbial communities can speedily and flexibly convert their components and functional repertoires following modern dietary challenges. Examples of this conversion could be supported by studies showing that a high HFD intake would increase the abundance rates of AIEC and Clostridioides difficile, decrease the abundance rates of Akkermansia muciniphila, and reduce the abundance rates of both Firmicutes and Bacteroidetes [96]. The clinical trial devised by Fritsch et al. [97] revealed that UC patients treated with a low-fat, high-fiber diet experienced a reduction in inflammation and an increase in the abundance of Bacteroidetes. Several studies have shown that these gut microbiotas and their metabolites are strongly correlated with IBD by potentially affecting intestinal immunity (Figure 2).
This entry is adapted from the peer-reviewed paper 10.3390/biom13060905
References
- Fiocchi, C. Inflammatory bowel disease: Etiology and pathogenesis. Gastroenterology 1998, 115, 182–205.
- Lin, L.; Li, Y.; Zhou, G.; Wang, Y.; Li, L.; Han, J.; Chen, M.; He, Y.; Zhang, S. Multi-Omics Analysis of Western-style Diet Increased Susceptibility to Experimental Colitis in Mice. J. Inflamm. Res. 2022, 15, 2523–2537.
- Levine, A.; Boneh, R.S.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738.
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11, 1033.
- Tong, Y.; Gao, H.; Qi, Q.; Liu, X.; Li, J.; Gao, J.; Li, P.; Wang, Y.; Du, L.; Wang, C. High Fat Diet, Gut Microbiome and Gastro-intestinal Cancer. Theranostics 2021, 11, 5889–5910.
- Molodecky, N.A.; Soon, S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42, quiz e30.
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778.
- Bilotta, A.J.; Cong, Y. Gut Microbiota Metabolite Regulation of Host Defenses at Mucosal Surfaces: Implication in Precision Medicine. Precis. Clin. Med. 2019, 2, 110–119.
- El-Salhy, M.; Solomon, T.; Hausken, T.; Gilja, O.H.; Hatlebakk, J.G. Gastrointestinal neuroendocrine peptides/amines in inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 5068–5085.
- Pradhan, G.; Samson, S.L.; Sun, Y. Ghrelin: Much More Than a Hunger Hormone. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 619–624.
- Waseem, T.; Duxbury, M.; Ito, H.; Ashley, S.W.; Robinson, M.K. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery 2008, 143, 334–342.
- Gonzalez–Rey, E.; Chorny, A.; Delgado, M. Therapeutic Action of Ghrelin in a Mouse Model of Colitis. Gastroenterology 2006, 130, 1707–1720.
- Maduzia, D.; Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Galazka, K.; Dembinski, A. The influence of pretreatment with ghrelin on the development of acetic-acid-induced colitis in rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2015, 66, 875–885.
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Gałązka, K.; Bonior, J.; Jaworek, J.; Bartuś, K.; Gil, K.; et al. Exogenous Ghrelin Accelerates the Healing of Acetic Acid-Induced Colitis in Rats. Int. J. Mol. Sci. 2016, 17, 1455.
- Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Gałązka, K.; Cieszkowski, J.; Bonior, J.; Jaworek, J.; Pihut, M.; et al. The Influence of Ghrelin on the Development of Dextran Sodium Sulfate-Induced Colitis in Rats. Biomed. Res. Int. 2015, 2015, 718314.
- Warzecha, Z.; Ceranowicz, D.; Dembiński, A.; Ceranowicz, P.; Cieszkowski, J.; Kuwahara, A.; Kato, I.; Konturek, P.C. Ghrelin accelerates the healing of cysteamine-induced duodenal ulcers in rats. Experiment 2012, 18, BR181–BR187.
- Warzecha, Z.; Kownacki, P.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Dembinski, A. Ghrelin accelerates the healing of oral ulcers in non-sialoadenectomized and sialoadenectomized rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2013, 64, 657–668.
- Stępniowska, A.; Tutaj, K.; Juśkiewicz, J.; Ognik, K. Effect of a high-fat diet and chromium on hormones level and Cr retention in rats. J. Endocrinol. Investig. 2022, 45, 527–535.
- Kellard, J.A.; Rorsman, N.J.; Hill, T.G.; Armour, S.L.; van de Bunt, M.; Rorsman, P.; Knudsen, J.G.; Briant, L.J. Reduced somatostatin signalling leads to hypersecretion of glucagon in mice fed a high-fat diet. Mol. Metab. 2020, 40, 101021.
- Liu, R.; Wei, N.; Guo, W.; Qiang, O.; Li, X.; Ou, Y.; Huang, W.; Tang, C.W. Octreotide alleviates obesity by reducing intestinal glucose absorption and inhibiting low-grade inflammation. Eur. J. Nutr. 2013, 52, 1067–1075.
- Ameri, P.; Ferone, D. Diffuse Endocrine System, Neuroendocrine Tumors and Immunity: What’s New? Neuroendocrinology 2012, 95, 267–276.
- Chen, Y.; Mu, J.; Zhu, M.; Mukherjee, A.; Zhang, H. Transient Receptor Potential Channels and Inflammatory Bowel Disease. Front. Immunol. 2020, 11, 180.
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434.
- Libertucci, J.; Dutta, U.; Kaur, S.; Jury, J.; Rossi, L.; Fontes, M.E.; Shajib, M.S.; Khan, W.I.; Surette, M.G.; Verdu, E.F.; et al. Inflammation-related differences in mucosa-associated microbiota and intestinal barrier function in colonic Crohn’s disease. Am. J. Physiol. Liver Physiol. 2018, 315, G420–G431.
- Arnott, I.D.; Kingstone, K.; Ghosh, S. Abnormal Intestinal Permeability Predicts Relapse in Inactive Crohn Disease. Scand. J. Gastroenterol. 2000, 35, 1163–1169.
- Choy, M.C.; Visvanathan, K.; De Cruz, P. An Overview of the Innate and Adaptive Immune System in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 2–13.
- Munkholm, P.; Langholz, E.; Hollander, D.; Thornberg, K.; Orholm, M.; Katz, K.D.; Binder, V. Intestinal Permeability in Patients with Crohn’s Disease and Ulcerative Colitis and Their First Degree Relatives. Gut 1994, 35, 68–72.
- Sartor, R.B.; Wu, G.D. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology 2017, 152, 327–339.e4.
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015, 37, 47–55.
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota Metabolite Short Chain Fatty Acids, Gpcr, and Inflammatory Bowel Diseases. J. Gastroenterol. 2017, 52, 1–8.
- Mazzarella, G.; Perna, A.; Marano, A.; Lucariello, A.; Rotondi Aufiero, V.; Sorrentino, A.; Melina, R.; Guerra, G.; Taccone, F.S.; Iaquinto, G.; et al. Pathogenic Role of Associated Adherent-Invasive Escherichia coli in Crohn’s Disease. J. Cell Physiol. 2017, 232, 2860–2868.
- Ma, C.; Vasu, R.; Zhang, H. The Role of Long-Chain Fatty Acids in Inflammatory Bowel Disease. Mediat. Inflamm. 2019, 2019, 1–10.
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834.
- Mankertz, J.; Schulzke, J.D. Altered Permeability in Inflammatory Bowel Disease: Pathophysiology and Clinical Implications. Curr. Opin. Gastroenterol. 2007, 23, 379–383.
- Chen, Y.; Cui, W.; Li, X.; Yang, H. Interaction Between Commensal Bacteria, Immune Response and the Intestinal Barrier in Inflammatory Bowel Disease. Front. Immunol. 2021, 12, 761981.
- Söderholm, J.D.; Peterson, K.H.; Olaison, G.; Franzén, L.E.; Weström, B.; Magnusson, K.E.; Sjödahl, R. Epithelial Perme-ability to Proteins in the Noninflamed Ileum of Crohn’s Disease? Gastroenterology 1999, 117, 65–72.
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91.
- Lu, Y.; Li, X.; Liu, S.; Zhang, Y.; Zhang, D. Toll-like Receptors and Inflammatory Bowel Disease. Front. Immunol. 2018, 9, 72.
- Shao, B.Z.; Xu, Z.Q.; Han, B.Z.; Su, D.F.; Liu, C. Nlrp3 Inflammasome and Its Inhibitors: A Review. Front. Pharmacol. 2015, 6, 262.
- Su, Y.R.; Hong, Y.P.; Mei, F.C.; Wang, C.Y.; Li, M.; Zhou, Y.; Zhao, K.L.; Yu, J.; Wang, W.X. High-Fat Diet Aggravates the Intestinal Barrier Injury via TLR4-RIP3 Pathway in a Rat Model of Severe Acute Pancreatitis. Mediat. Inflamm. 2019, 2019, 2512687.
- Cremonini, E.; Wang, Z.; Bettaieb, A.; Adamo, A.M.; Daveri, E.; Mills, D.A.; Kalanetra, K.M.; Haj, F.G.; Karakas, S.; Oteiza, P.I. (-)-Epicatechin protects the intestinal barrier from high fat diet-induced permeabilization: Implications for steatosis and insulin resistance. Redox Biol. 2018, 14, 588–599.
- Kim, K.-A.; Gu, W.; Lee, I.-A.; Joh, E.-H.; Kim, D.-H. High Fat Diet-Induced Gut Microbiota Exacerbates Inflammation and Obesity in Mice via the TLR4 Signaling Pathway. PLoS ONE 2012, 7, e47713.
- Wang, L.; Gong, Z.; Zhang, X.; Zhu, F.; Liu, Y.; Jin, C.; Du, X.; Xu, C.; Chen, Y.; Cai, W.; et al. Gut microbial bile acid metabolite skews macrophage polarization and contributes to high-fat diet-induced colonic inflammation. Gut Microbes 2020, 12, 1819155.
- Reynolds, C.M.; McGillicuddy, F.C.; Harford, K.A.; Finucane, O.M.; Mills, K.H.G.; Roche, H.M. Dietary saturated fatty acids prime the NLRP3 inflammasome via TLR4 in dendritic cells-implications for diet-induced insulin resistance. Mol. Nutr. Food Res. 2012, 56, 1212–1222.
- Snodgrass, R.G.; Huang, S.; Choi, I.-W.; Rutledge, J.C.; Hwang, D.H. Inflammasome-Mediated Secretion of IL-1β in Human Monocytes through TLR2 Activation; Modulation by Dietary Fatty Acids. J. Immunol. 2013, 191, 4337–4347.
- Maynard, C.L.; Weaver, C.T. Intestinal Effector T Cells in Health and Disease. Immunity 2009, 31, 389–400.
- Elson, C.O.; Cong, Y.; McCracken, V.J.; Dimmitt, R.A.; Lorenz, R.G.; Weaver, C.T. Experimental Models of Inflammatory Bowel Disease Reveal Innate, Adaptive, and Regulatory Mechanisms of Host Dialogue with the Microbiota. Immunol. Rev. 2005, 206, 260–276.
- Fuss, I.J.; Neurath, M.F.; Boirivant, M.; Fiocchi, C.; Strober, W. Disparate Cd4+ Lamina Propria (Lp) Lymphokine Secretion Profiles in Inflammatory Bowel Disease. Crohn’s Disease Lp Cells Manifest Increased Secretion of Ifn-Gamma, Whereas Ulcerative Colitis Lp Cells Manifest Increased Secretion of Il-5. J. Immunol. 1996, 157, 1261–1270.
- Yang, W.; Yu, T.; Cong, Y. CD4+ T cell metabolism, gut microbiota, and autoimmune diseases: Implication in precision medicine of autoimmune diseases. Precis. Clin. Med. 2022, 5, pbac018.
- Ma, X.; Torbenson, M.; Hamad, A.R.A.; Soloski, M.J.; Li, Z. High-fat diet modulates non-CD1d-restricted natural killer T cells and regulatory T cells in mouse colon and exacerbates experimental colitis. Clin. Exp. Immunol. 2008, 151, 130–138.
- Park, C.; Cheung, K.P.; Limon, N.; Costanzo, A.; Barba, C.; Miranda, N.; Gargas, S.; Johnson, A.M.; Olefsky, J.M.; Jameson, J.M. Obesity Modulates Intestinal Intraepithelial T Cell Persistence, Cd103 and Ccr9 Expression, and Outcome in Dextran Sulfate Sodium-Induced Colitis. J. Immunol. 2019, 203, 3427–3435.
- Okada, Y.; Tsuzuki, Y.; Sato, H.; Narimatsu, K.; Hokari, R.; Kurihara, C.; Watanabe, C.; Tomita, K.; Komoto, S.; Kawaguchi, A.; et al. Trans fatty acids exacerbate dextran sodium sulphate-induced colitis by promoting the up-regulation of macrophage-derived proinflammatory cytokines involved in T helper 17 cell polarization. Clin. Exp. Immunol. 2013, 174, 459–471.
- Ding, N.; Zhang, X.; Di Zhang, X.; Jing, J.; Liu, S.S.; Mu, Y.P.; Peng, L.L.; Yan, Y.J.; Xiao, G.M.; Bi, X.; et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 2020, 69, 1608–1619.
- Rohr, M.; Narasimhulu, C.A.; Keewan, E.; Hamid, S.; Parthasarathy, S. The dietary peroxidized lipid, 13-HPODE, promotes intestinal inflammation by mediating granzyme B secretion from natural killer cells. Food Funct. 2020, 11, 9526–9534.
- Saez, A.; Gomez-Bris, R.; Herrero-Fernandez, B.; Mingorance, C.; Rius, C.; Gonzalez-Granado, J.M. Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 7618.
- Babu, S.T.; Niu, X.; Raetz, M.; Savani, R.C.; Hooper, L.V.; Mirpuri, J. Maternal High-Fat Diet Results in Microbio-ta-Dependent Expansion of Ilc3s in Mice Offspring. JCI Insight 2018, 3, e99223.
- Mxinwa, V.; Nkambule, B.B.; Nyambuya, T.M.; Dludla, P.V. Expression of Caspase-3 in Circulating Innate Lymphoid Cells Subtypes Is Altered by Treatment with Metformin and Fluvastatin in High-Fat Diet Fed C57BL/6 Mice. Cells 2022, 11, 1430.
- Cui, J.; Xiao, Y.; Shi, Y.-H.; Wang, B.; Le, G.-W. Lipoic acid attenuates high-fat-diet–induced oxidative stress and B-cell–related immune depression. Nutrition 2012, 28, 275–280.
- Gurzell, E.A.; Teague, H.; Harris, M.; Clinthorne, J.; Shaikh, S.R.; Fenton, J.I. DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function. J. Leukoc. Biol. 2013, 93, 463–470.
- Iftikhar, R.; Penrose, H.M.; King, A.N.; Kim, Y.; Ruiz, E.; Kandil, E.; Machado, H.L.; Savkovic, S.D. FOXO3 Expression in Macrophages Is Lowered by a High-Fat Diet and Regulates Colonic Inflammation and Tumorigenesis. Metabolites 2022, 12, 250.
- Senokuchi, T.; Liang, C.P.; Seimon, T.A.; Han, S.; Matsumoto, M.; Banks, A.S.; Paik, J.H.; DePinho, R.A.; Accili, D.; Tabas, I.; et al. Forkhead Transcription Factors (Foxos) Promote Apoptosis of Insulin-Resistant Macrophages During Cholester-ol-Induced Endoplasmic Reticulum Stress. Diabetes 2008, 57, 2967–2976.
- Chen, H.; Wu, X.; Xu, C.; Lin, J.; Liu, Z. Dichotomous roles of neutrophils in modulating pathogenic and repair processes of inflammatory bowel diseases. Precis. Clin. Med. 2021, 4, 246–257.
- Guo, H.; Diao, N.; Yuan, R.; Chen, K.; Geng, S.; Li, M.; Li, L. Subclinical-Dose Endotoxin Sustains Low-Grade Inflammation and Exacerbates Steatohepatitis in High-Fat Diet–Fed Mice. J. Immunol. 2016, 196, 2300–2308.
- Bibi, S.; Kang, Y.; Du, M.; Zhu, M.-J. Maternal high-fat diet consumption enhances offspring susceptibility to DSS-induced colitis in mice. Obesity 2017, 25, 901–908.
- Pérez, M.M.; Martins, L.M.; Dias, M.S.; Pereira, C.A.; Leite, J.A.; Gonçalves, E.C.; de Almeida, P.Z.; de Freitas, E.N.; Tostes, R.C.; Ramos, S.G.; et al. Interleukin-17/Interleukin-17 Receptor Axis Elicits Intestinal Neutrophil Migration, Restrains Gut Dysbiosis and Lipopolysaccharide Translocation in High-Fat Diet-Induced Metabolic Syndrome Model. Immunology 2019, 156, 339–355.
- Yoshida, H.; Kishikawa, H.; Hirokawa, M.; Nakamizo, H.; Nakatsumi, R.C.; Suzuki, H.; Saito, H.; Miura, S.; Ishii, H. Fatty Acids Enhance GRO/CINC-1 and Interleukin-6 Production in Rat Intestinal Epithelial Cells. J. Nutr. 2001, 131, 2943–2950.
- Gruber, L.; Kisling, S.; Lichti, P.; Martin, F.-P.; May, S.; Klingenspor, M.; Lichtenegger, M.; Rychlik, M.; Haller, D. High Fat Diet Accelerates Pathogenesis of Murine Crohn’s Disease-Like Ileitis Independently of Obesity. PLoS ONE 2013, 8, e71661.
- Cheng, L.; Jin, H.; Qiang, Y.; Wu, S.; Yan, C.; Han, M.; Xiao, T.; Yan, N.; An, H.; Zhou, X.; et al. High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. Int. Immunopharmacol. 2016, 40, 1–10.
- Wien, M.; Rajaram, S.; Oda, K.; Sabaté, J. Decreasing the Linoleic Acid to Alpha-Linolenic Acid Diet Ratio Increases Eicosapentaenoic Acid in Erythrocytes in Adults. Lipids 2010, 45, 683–692.
- Belluzzi, A.; Boschi, S.; Brignola, C.; Munarini, A.; Cariani, G.; Miglio, F. Polyunsaturated fatty acids and inflammatory bowel disease. Am. J. Clin. Nutr. 2000, 71, 339S–342S.
- John, S.; Luben, R.; Shrestha, S.S.; Welch, A.; Khaw, K.-T.; Hart, A.R. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: A UK prospective cohort study. Eur. J. Gastroenterol. Hepatol. 2010, 22, 602–606.
- Uchiyama, K.; Nakamura, M.; Odahara, S.; Koido, S.; Katahira, K.; Shiraishi, H.; Ohkusa, T.; Fujise, K.; Tajiri, H. N-3 Poly-unsaturated Fatty Acid Diet Therapy for Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2010, 16, 1696–1707.
- Beguin, P.; Errachid, A.; Larondelle, Y.; Schneider, Y.-J. Effect of polyunsaturated fatty acids on tight junctions in a model of the human intestinal epithelium under normal and inflammatory conditions. Food Funct. 2013, 4, 923–931.
- Charpentier, C.; Chan, R.; Salameh, E.; Mbodji, K.; Ueno, A.; Coëffier, M.; Guérin, C.; Ghosh, S.; Savoye, G.; Marion-Letellier, R. Dietary n-3 PUFA May Attenuate Experimental Colitis. Mediat. Inflamm. 2018, 2018, 8430614.
- Hawthorne, A.B.; Daneshmend, T.K.; Hawkey, C.J.; Belluzzi, A.; Everitt, S.J.; Holmes, G.K.; Malkinson, C.; Shaheen, M.Z.; EWillars, J. Treatment of ulcerative colitis with fish oil supplementation: A prospective 12 month randomised controlled trial. Gut 1992, 33, 922–928.
- Teague, H.; Rockett, B.D.; Harris, M.; Brown, D.A.; Shaikh, S.R. Dendritic Cell Activation, Phagocytosis and Cd69 Ex-pression on Cognate T Cells Are Suppressed by N-3 Long-Chain Polyunsaturated Fatty Acids. Immunology 2013, 139, 386–394.
- Ly, L.H.; Smith, R.; Switzer, K.C.; Chapkin, R.S.; McMurray, D.N. Dietary Eicosapentaenoic Acid Modulates Ctla-4 Ex-pression in Murine Cd4+ T-Cells. Prostaglandins Leukot Essent Fat. Acids 2006, 74, 29–37.
- Pearl, D.S.; Masoodi, M.; Eiden, M.; Brümmer, J.; Gullick, D.; Mckeever, T.M.; Whittaker, M.A.; Nitch-Smith, H.; Brown, J.F.; Shute, J.K.; et al. Altered colonic mucosal availability of n-3 and n-6 polyunsaturated fatty acids in ulcerative colitis and the relationship to disease activity. J. Crohn’s Colitis 2014, 8, 70–79.
- Krause, P.; Bruckner, M.; Uermösi, C.; Singer, E.; Groettrup, M.; Legler, D.F. Prostaglandin E(2) Enhances T-Cell Prolifer-ation by Inducing the Costimulatory Molecules Ox40l, Cd70, and 4-1bbl on Dendritic Cells. Blood 2009, 113, 2451–2460.
- Arimoto-Miyamoto, K.; Kadowaki, N.; Kitawaki, T.; Iwata, S.; Morimoto, C.; Uchiyama, T. Optimal stimulation for CD70 induction on human monocyte-derived dendritic cells and the importance of CD70 in naive CD4+T-cell differentiation. Immunology 2010, 130, 137–149.
- Monk, J.M.; Hou, T.Y.; Turk, H.F.; Weeks, B.; Wu, C.; McMurray, D.N.; Chapkin, R.S. Dietary n-3 Polyunsaturated Fatty Acids (PUFA) Decrease Obesity-Associated Th17 Cell-Mediated Inflammation during Colitis. PLoS ONE 2012, 7, e49739.
- Monk, J.M.; Jia, Q.; Callaway, E.; Weeks, B.; Alaniz, R.C.; McMurray, D.N.; Chapkin, R.S. Th17 Cell Accumulation Is De-creased During Chronic Experimental Colitis by (N-3) Pufa in Fat-1 Mice. J. Nutr. 2012, 142, 117–124.
- Zhang, W.; Zhu, S. Gut metabolites: Make orphans adopted. Precis. Clin. Med. 2019, 2, 87–89.
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor–Dependent Repression of Claudin-2. J. Immunol. 2017, 199, 2976–2984.
- Hatayama, H.; Iwashita, J.; Kuwajima, A.; Abe, T. The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochem. Biophys. Res. Commun. 2007, 356, 599–603.
- Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018, 11, 752–762.
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018, 9, 3555.
- Park, J.; Kim, M.; Kang, S.; Jannasch, A.; Cooper, B.; Patterson, J.; Kim, C. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 2015, 8, 80–93.
- Haidinger, M.; Poglitsch, M.; Geyeregger, R.; Kasturi, S.; Zeyda, M.; Zlabinger, G.J.; Pulendran, B.; Hörl, W.H.; Säemann, M.D.; Weichhart, T. A Versatile Role of Mammalian Target of Rapamycin in Human Dendritic Cell Function and Differentiation. J. Immunol. 2010, 185, 3919–3931.
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.e437.
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252.
- Vinolo, M.A.R.; Rodrigues, H.G.; Hatanaka, E.; Hebeda, C.B.; Farsky, S.H.P.; Curi, R. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin. Sci. 2009, 117, 331–338.
- Vinolo, M.A.R.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855.
- Weingarden, A.R.; Vaughn, B.P. Intestinal Microbiota, Fecal Microbiota Transplantation, and Inflammatory Bowel Disease. Gut Microbes 2017, 8, 238–252.
- Ding, X.; Li, Q.; Li, P.; Zhang, T.; Cui, B.; Ji, G.; Lu, X.; Zhang, F. Long-Term Safety and Efficacy of Fecal Microbiota Transplant in Active Ulcerative Colitis. Drug Saf. 2019, 42, 869–880.
- Rapozo, D.C.; Bernardazzi, C.; de Souza, H.S. Diet and Microbiota in Inflammatory Bowel Disease: The Gut in Dis-harmony. World J. Gastroenterol. 2017, 23, 2124–2240.
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30.
This entry is offline, you can click here to edit this entry!
