Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Milk is a unique natural source of exosomes available in semi-preparative and preparative quantities. Milk exosomes are highly resistant to the harsh conditions of the gastrointestinal tract. In vitro studies have demonstrated that milk exosomes have an affinity to epithelial cells, are digested by cells by endocytosis mechanism, and can be used for oral delivery. With milk exosome membranes containing hydrophilic and hydrophobic components, exosomes can be loaded with hydrophilic and lipophilic drugs.
- exosomes
- milk exosomes
- milk vesicles
- extracellular vesicles
- drug delivery
1. Milk Exosomes
Exosomes are membrane vesicles of cellular origin released by cells of various types and found in almost all physiological fluids. Exosomes have a diameter of 40 to 120 nm and are coated with a phospholipid bilayer derived from the membrane of the cell of origin [1][2]. Exosomes have been shown to be involved in intercellular communication and transfer their content from the parent cell to the recipient cells [3][4]. Exosomes are known to contain various biologically important molecules: proteins, lipids, DNA, mRNA, microRNA, and others [3].
Exosomes have been found in blood, urine, saliva, milk, ascites fluid, and other biological fluids [5]. The high biocompatibility of exosomes makes them an attractive alternative tool for the delivery of therapeutically relevant drugs, albeit poorly explored to date [3][6].
The benefits of using exosomes are: (1) low immunogenicity [7]; (2) easy entry into cells due to the natural origin and biocompatible structure allowing for more efficient cargo delivery [8]; (3) robust membrane with a potential to protect therapeutically relevant molecules from degradation [9]; (4) capability for long-term circulation in the body [8]; (5) the potential for their membrane to be artificially modified for targeted delivery, enabling tissue-specific or cell-specific distribution [10]. (6) As for milk exosomes, their advantage is that milk is an inexpensive source of exosomes, compared with culture fluid, and safe, compared with blood and other biological fluids, available on a preparative scale [11]. Moreover, exosomes can cross the blood–brain barrier [12][13] and the placental barrier [14][15]. Their ability to cross the blood–brain barrier can be used to develop delivery systems to brain tissue, for example, in Alzheimer’s and Parkinson’s disease [16].
Exosomes have been found in almost all body fluids [2]. Many researchers use exosomes isolated from cell cultures [17][18], although the yield of exosomes from this source is rather low. Moreover, there is a possibility of contamination by vesicles from the serum used for cell cultivation. Another concern is the safety of exosomes derived from cancer and immortalized cell lines to be used in therapy [19].
Milk is a unique natural source of exosomes available in semi-preparative and preparative quantities. Milk exosomes are highly resistant to the harsh conditions of the gastrointestinal tract, with in vitro studies indicating their affinity for epithelial cells, cellular uptake by the endocytosis mechanism, and, importantly, their potential to be used for oral delivery [20]. Due to their membrane containing hydrophilic and hydrophobic components, milk exosomes can be loaded with hydrophilic and lipophilic drugs [21].
The mechanism of exosome formation involves a stage of endocytosis and release by intraluminal budding of multivesicular bodies with the cell membrane of mammary epithelial cells. Milk exosomes have been reported to contain CD9, CD63, CD81, CD82, HSP70, HSP90, Alix, TSG101, annexin, and Rab GTPases [22][23]. Previously, researchers described in detail the proteins, lipids, and nucleic acids that makeup milk exosomes [11][24]. Various sources have reported hundreds and thousands of proteins and mRNAs in milk exosomes. According to the data on protein content in well-purified exosome preparations from horse milk [25][26], numerous proteins, such as caseins, cannot be intrinsic components of milk exosomes but are co-purified with exosomes by crude purification methods. Thus, insufficiently purified preparations can be credited with at least part of the biological functions of milk exosomes. Thus, further studies should be conducted on highly purified preparations of milk exosomes [11].
Exosomes were first described in human milk in 2007 [27]. Nowadays, milk exosomes are regarded as a promising alternative to exosomes derived from cell cultures, with great potential as drug delivery vehicles [20][28]. One of the advantages of exosomes isolated from milk is the high availability of milk, which no other exosome sources can compete [11]. Most global population regularly consumes significant amounts of milk and dairy products, making dairy exosomes a potentially safe delivery vehicle [29]. Bovine milk exosomes were demonstrated to exhibit low systemic immunogenicity in vivo [20][30]. In addition, milk exosomes were reported to be highly resistant to the harsh conditions of the gastrointestinal tract, capable of crossing biological barriers and reaching peripheral tissues suggesting milk exosomes as promising agents for oral minimally invasive delivery [29][31][32]. The use of milk exosomes is considered for liquid biopsy of breast cancer [33]. An increased expression of TGFβ2 in milk exosomes may be associated with the development of breast cancer [34]. In addition, changes in the expression of microRNA [35] and protein components [36] of milk exosomes may be associated with inflammation of the mammary gland (mastitis) in cows. Researchers believe that the use of milk exosomes as an object for liquid biopsy is of little value for humans due to the relatively low detection rate of breast cancer in lactating women, the absence of troubles with a diagnosis of mammary gland inflammation, and the relatively low duration of lactation [37]. Although it cannot be ruled out, the short lactation period explains, in turn, a small number of publications on the use of milk exosomes for diagnosing breast pathologies.
2. Milk Exosomes—Promising Agents for Oral Delivery
Milk exosomes have several advantages compared to exosomes from other biological fluids, including natural compatibility for [20]. On the one hand, oral delivery is one of the least invasive ways to deliver therapeutically important biomolecules. On the other hand, the aggressive environment of the gastrointestinal tract poses significant problems for their delivery [38][39].
In vitro studies show that milk-derived exosomes have an affinity for intestinal epithelial cells [40][41] and can enter the cells by endocytosis [42][43]. Therapeutic drugs encapsulated in exosomes can be delivered orally into the bloodstream of laboratory animals in a bioactive form [44]. For example, milk exosomes and their microRNAs have been shown to be stable in gastric juice and pancreatic secretion [40][45] and stable when incubated with saliva or bile [41].
Orally administered milk exosomes exhibit a wide bio-distribution, with labeled exosomes found in the liver, spleen, kidneys, pancreas, ovaries, lungs, heart, brain, and colon [29][32][46]. The greatest amount of milk exosomes is accumulated in the liver and spleen due to the phagocytic activity of Kupffer macrophages in these organs [47]. Histological evaluations confirm exosome accumulation does not cause tissue damage or changes in liver tissue structure [48].
A general diagram of the use of milk exosomes as a delivery vehicle from isolation to imaging is shown in Figure 1.
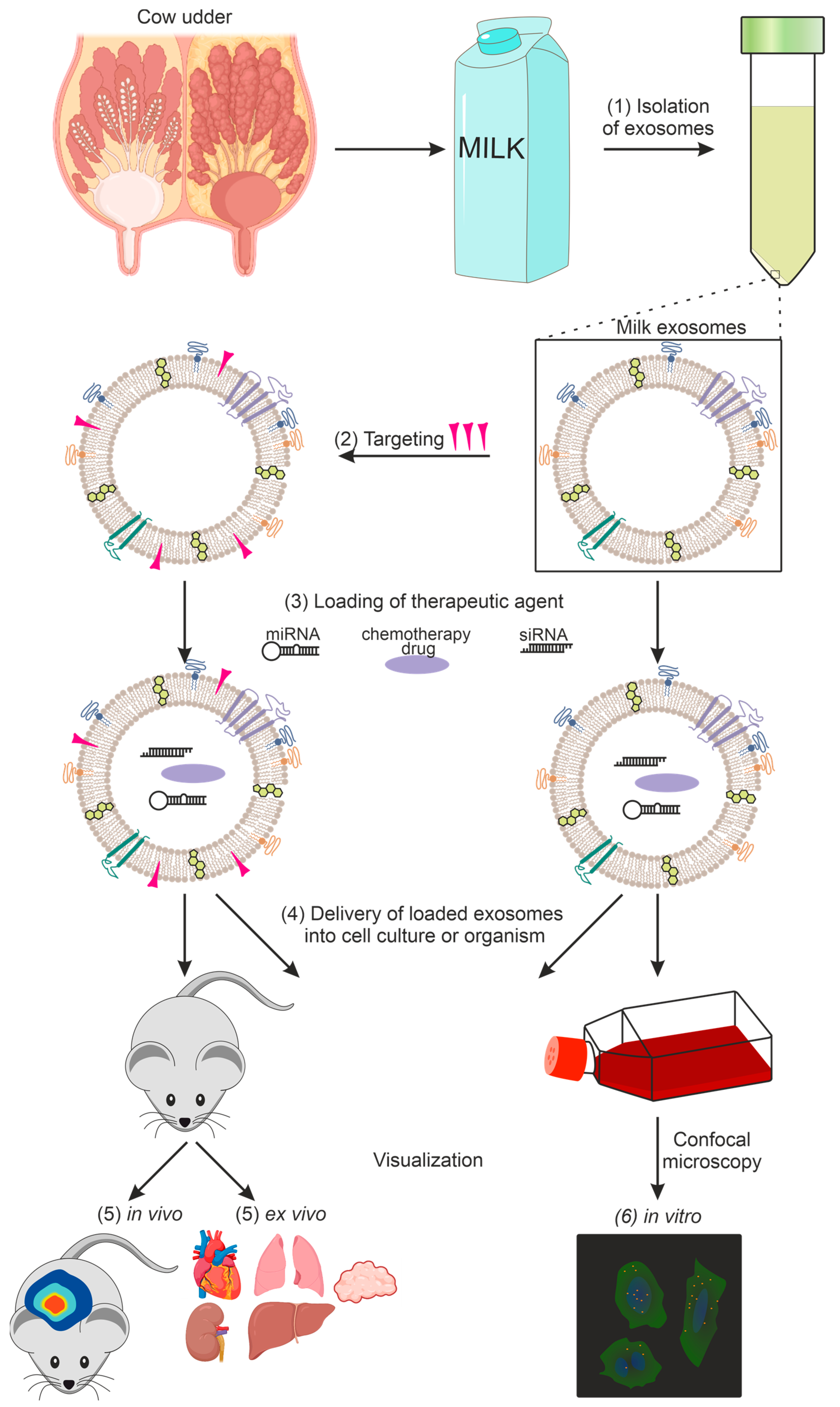
Figure 1. Using exosomes as a delivery vehicle for therapeutic agents: (1) exosome isolation, (2) targeting of exosomes, (3) loading of the therapeutic agent, (4) delivery of loaded exosomes into cell culture or organism, (5) in vivo and ex vivo imaging, (6) in vitro imaging.
Comparison of the bioavailability of radiolabeled goat milk exosomes with different routes of administration shows that the intravenous route of administration led to a rapid accumulation of exosomes in the liver; intraperitoneal administration led to the slow appearance of exosomes in the bloodstream, and their accumulation into the thyroid gland and lungs; intranasal administration led to the entry of exosomes into the brain [49].
3. Isolation of Milk Exosomes
Milk contains large amounts of proteins and lipids (including fats). It is necessary to remove these impurities before isolating exosomes, since fat, cell debris, and free proteins significantly hamper the production of pure exosome preparations. Methods for isolating and purifying milk exosomes include differential centrifugation, tangential flow filtration, density gradient centrifugation, ultracentrifugation, immune affinity capture, gel filtration, and other types of chromatography, while microfluidic techniques and some other methods have not yet been used for exosome isolation from milk [50][51]. Standard approaches for isolating and purifying exosomes from milk and other biological fluids are shown in Table 1.
Table 1. Extraction and purification of exosomes from milk and other biological fluids: advantages and disadvantages.
| Methods of Isolation | Principle of Isolation | Advantages | Disadvantages |
|---|---|---|---|
| Centrifugation [52][53] | Differential centrifugation, density gradient centrifugation, isoelectric deposition | Method simplicity, high yield | Time-consuming, low purity, exosomes can be destroyed, high cost of ultracentrifuges |
| Ultrafiltration [54] | By vesicle size | Low cost, no expensive equipment required | Low purity |
| Immunological [25][26] | Binding of antibodies to specific exosome markers | High purity, high specificity, low equipment cost, easy to operate | High reagent cost, low yield |
| Microfluidic devices [55] | Size, density, surface antigens | High purity, portability, easy to operate, time-saving, automation, speed of extraction | Small sample volume, no method validation Small sample volume, no method validation |
| Chromatography [52] | Gel filtration, affinity chromatography | High purity, scalability | Requires special equipment, sorbents |
| Co-precipitation [56] | Precipitation with commercial reagents | Easy to use, time-saving | Expensive, low purity, small sample volume, difficult to scale |
According to MISEV2018, existing exosome isolation methods are classified by recovery and specificity, and no single high recovery and high specificity method have been developed so far. Therefore, a combination of several methods is usually used to isolate pure exosome preparations that do not contain impurities of co-precipitating structures, with the first method being high recovery and the subsequent methods being high specificity ones [57].
This entry is adapted from the peer-reviewed paper 10.3390/ijms241210194
References
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228.
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289.
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977.
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232.
- Simpson, R.J.; Lim, J.W.; Moritz, R.L.; Mathivanan, S. Exosomes: Proteomic Insights and Diagnostic Potential. Expert Rev. Proteom. 2009, 6, 267–283.
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering Exosomes for Targeted Drug Delivery. Theranostics 2021, 11, 3183–3195.
- Pirisinu, M.; Pham, T.C.; Zhang, D.X.; Hong, T.N.; Nguyen, L.T.; Le, M.T. Extracellular Vesicles as Natural Therapeutic Agents and Innate Drug Delivery Systems for Cancer Treatment: Recent Advances, Current Obstacles, and Challenges for Clinical Translation. Semin. Cancer Biol. 2022, 80, 340–355.
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular Vesicles as a Next-Generation Drug Delivery Platform. Nat. Nanotechnol. 2021, 16, 748–759.
- Ullah, M.; Kodam, S.P.; Mu, Q.; Akbar, A. Microbubbles versus Extracellular Vesicles as Therapeutic Cargo for Targeting Drug Delivery. ACS Nano 2021, 15, 3612–3620.
- Yao, X.; Lyu, P.; Yoo, K.; Yadav, M.K.; Singh, R.; Atala, A.; Lu, B. Engineered Extracellular Vesicles as Versatile Ribonucleoprotein Delivery Vehicles for Efficient and Safe CRISPR Genome Editing. J. Extracell. Vesicles 2021, 10, e12076.
- Sedykh, S.; Kuleshova, A.; Nevinsky, G. Milk Exosomes: Perspective Agents for Anticancer Drug Delivery. Int. J. Mol. Sci. 2020, 21, 6646.
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of SiRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29, 341–345.
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407.
- Record, M. Intercellular Communication by Exosomes in Placenta: A Possible Role in Cell Fusion? Placenta 2014, 35, 297–302.
- Lee, B.; Saadeldin, I.; Oh, H.J. Embryonic–Maternal Cross-Talk via Exosomes: Potential Implications. Stem Cells Cloning Adv. Appl. 2015, 8, 103–107.
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as Drug Delivery Vehicles for Parkinson’s Disease Therapy. J. Control. Release 2015, 207, 18–30.
- Kooijmans, S.A.A.; Gitz-Francois, J.J.J.M.; Schiffelers, R.M.; Vader, P. Recombinant Phosphatidylserine-Binding Nanobodies for Targeting of Extracellular Vesicles to Tumor Cells: A Plug-and-Play Approach. Nanoscale 2018, 10, 2413–2426.
- Antes, T.J.; Middleton, R.C.; Luther, K.M.; Ijichi, T.; Peck, K.A.; Liu, W.J.; Valle, J.; Echavez, A.K.; Marbán, E. Targeting Extracellular Vesicles to Injured Tissue Using Membrane Cloaking and Surface Display. J. Nanobiotechnol. 2018, 16, 61.
- Whitford, W.; Guterstam, P. Exosome Manufacturing Status. Future Med. Chem. 2019, 11, 1225–1236.
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine Milk-Derived Exosomes for Drug Delivery. Cancer Lett. 2016, 371, 48–61.
- Li, Y.; Xing, L.; Wang, L.; Liu, X.; Wu, L.; Ni, M.; Zhou, Z.; Li, L.; Liu, X.; Huang, Y. Milk-Derived Exosomes as a Promising Vehicle for Oral Delivery of Hydrophilic Biomacromolecule Drugs. Asian J. Pharm. Sci. 2023, 18, 100797.
- De la Torre Gomez, C.; Goreham, R.V.; Bech Serra, J.J.; Nann, T.; Kussmann, M. “Exosomics”—A Review of Biophysics, Biology and Biochemistry of Exosomes with a Focus on Human Breast Milk. Front. Genet. 2018, 9, 92.
- Adriano, B.; Cotto, N.M.; Chauhan, N.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Milk Exosomes: Nature’s Abundant Nanoplatform for Theranostic Applications. Bioact. Mater. 2021, 6, 2479–2490.
- Sedykh, S.E.; Burkova, E.E.; Purvinsh, L.V.; Klemeshova, D.A.; Ryabchikova, E.I.; Nevinsky, G.A. Milk Exosomes: Isolation, Biochemistry, Morphology, and Perspectives of Use. In Extracellular Vesicles and Their Importance in Human Health; Intech Open: London, UK, 2020.
- Sedykh, S.E.; Purvinsh, L.V.; Burkova, E.E.; Dmitrenok, P.S.; Ryabchikova, E.I.; Nevinsky, G.A. Analysis of Proteins and Peptides of Highly Purified CD9+ and CD63+ Horse Milk Exosomes Isolated by Affinity Chromatography. Int. J. Mol. Sci. 2022, 23, 16106.
- Sedykh, S.E.; Purvinish, L.V.; Burkova, E.E.; Dmitrenok, P.S.; Vlassov, V.V.; Ryabchikova, E.I.; Nevinsky, G.A. Analysis of Peptides and Small Proteins in Preparations of Horse Milk Exosomes, Purified on Anti-CD81-Sepharose. Int. Dairy J. 2021, 117, 104994.
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filén, J.-J.; Lahesmaa, R.; Norman, M.; Neve, E.P.A.; Scheynius, A.; Gabrielsson, S. Exosomes with Immune Modulatory Features Are Present in Human Breast Milk. J. Immunol. 2007, 179, 1969–1978.
- Plantz, P.E.; Patton, S.; Keenan, T.W. Further Evidence of Plasma Membrane Material in Skim Milk. J. Dairy Sci. 1973, 56, 978–983.
- Betker, J.L.; Angle, B.M.; Graner, M.W.; Anchordoquy, T.J. The Potential of Exosomes from Cow Milk for Oral Delivery. J. Pharm. Sci. 2019, 108, 1496–1505.
- Somiya, M.; Yoshioka, Y.; Ochiya, T. Biocompatibility of Highly Purified Bovine Milk-derived Extracellular Vesicles. J. Extracell. Vesicles 2018, 7, 1440132.
- Sadri, M.; Shu, J.; Kachman, S.D.; Cui, J.; Zempleni, J. Milk Exosomes and MiRNA Cross the Placenta and Promote Embryo Survival in Mice. Reproduction 2020, 160, 501–509.
- Manca, S.; Upadhyaya, B.; Mutai, E.; Desaulniers, A.T.; Cederberg, R.A.; White, B.R.; Zempleni, J. Milk Exosomes Are Bioavailable and Distinct MicroRNA Cargos Have Unique Tissue Distribution Patterns. Sci. Rep. 2018, 8, 11321.
- Halvaei, S.; Daryani, S.; Eslami-S., Z.; Samadi, T.; Jafarbeik-Iravani, N.; Bakhshayesh, T.O.; Majidzadeh-A., K.; Esmaeili, R. Exosomes in Cancer Liquid Biopsy: A Focus on Breast Cancer. Mol. Ther. Nucleic Acids 2018, 10, 131–141.
- Qin, W.; Tsukasaki, Y.; Dasgupta, S.; Mukhopadhyay, N.; Ikebe, M.; Sauter, E.R. Exosomes in Human Breast Milk Promote EMT. Clin. Cancer Res. 2016, 22, 4517–4524.
- Stefanon, B.; Cintio, M.; Sgorlon, S.; Scarsella, E.; Licastro, D.; Zecconi, A.; Colitti, M. Regulatory Role of MicroRNA of Milk Exosomes in Mastitis of Dairy Cows. Animals 2023, 13, 821.
- Reinhardt, T.A.; Sacco, R.E.; Nonnecke, B.J.; Lippolis, J.D. Bovine Milk Proteome: Quantitative Changes in Normal Milk Exosomes, Milk Fat Globule Membranes and Whey Proteomes Resulting from Staphylococcus Aureus Mastitis. J. Proteom. 2013, 82, 141–154.
- Helewa, M.; Lévesque, P.; Provencher, D.; Lea, R.H.; Rosolowich, V.; Shapiro, H.M.; Breast Disease Committee and Executive Committeee and Council; Society of Obstetricians and Gynaecologists of Canada. Breast Cancer, Pregnancy, and Breastfeeding. J. Obstet. Gynaecol. Can. 2002, 24, 164–180.
- Ahadian, S.; Finbloom, J.A.; Mofidfar, M.; Diltemiz, S.E.; Nasrollahi, F.; Davoodi, E.; Hosseini, V.; Mylonaki, I.; Sangabathuni, S.; Montazerian, H.; et al. Micro and Nanoscale Technologies in Oral Drug Delivery. Adv. Drug Deliv. Rev. 2020, 157, 37–62.
- Bakhru, S.H.; Furtado, S.; Morello, A.P.; Mathiowitz, E. Oral Delivery of Proteins by Biodegradable Nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 811–821.
- Liao, Y.; Du, X.; Li, J.; Lönnerdal, B. Human Milk Exosomes and Their MicroRNAs Survive Digestion In Vitro and Are Taken Up by Human Intestinal Cells. Mol. Nutr. Food Res. 2017, 61, 1700082.
- Rani, P.; Vashisht, M.; Golla, N.; Shandilya, S.; Onteru, S.K.; Singh, D. Milk MiRNAs Encapsulated in Exosomes Are Stable to Human Digestion and Permeable to Intestinal Barrier In Vitro. J. Funct. Foods 2017, 34, 431–439.
- Kusuma, R.J.; Manca, S.; Friemel, T.; Sukreet, S.; Nguyen, C.; Zempleni, J. Human Vascular Endothelial Cells Transport Foreign Exosomes from Cow’s Milk by Endocytosis. Am. J. Physiol. Physiol. 2016, 310, C800–C807.
- Wolf, T.; Baier, S.R.; Zempleni, J. The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells. J. Nutr. 2015, 145, 2201–2206.
- Benmoussa, A.; Lee, C.H.C.; Laffont, B.; Savard, P.; Laugier, J.; Boilard, E.; Gilbert, C.; Fliss, I.; Provost, P. Commercial Dairy Cow Milk MicroRNAs Resist Digestion under Simulated Gastrointestinal Tract Conditions. J. Nutr. 2016, 146, 2206–2215.
- Izumi, H.; Kosaka, N.; Shimizu, T.; Sekine, K.; Ochiya, T.; Takase, M. Bovine Milk Contains MicroRNA and Messenger RNA That Are Stable under Degradative Conditions. J. Dairy Sci. 2012, 95, 4831–4841.
- Samuel, M.; Fonseka, P.; Sanwlani, R.; Gangoda, L.; Chee, S.H.; Keerthikumar, S.; Spurling, A.; Chitti, S.V.; Zanker, D.; Ang, C.-S.; et al. Oral Administration of Bovine Milk-Derived Extracellular Vesicles Induces Senescence in the Primary Tumor but Accelerates Cancer Metastasis. Nat. Commun. 2021, 12, 3950.
- Almeida, J.P.M.; Chen, A.L.; Foster, A.; Drezek, R. In Vivo Biodistribution of Nanoparticles. Nanomedicine 2011, 6, 815–835.
- González, M.I.; González-Arjona, M.; Santos-Coquillat, A.; Vaquero, J.; Vázquez-Ogando, E.; de Molina, A.; Peinado, H.; Desco, M.; Salinas, B. Covalently Labeled Fluorescent Exosomes for In Vitro and In Vivo Applications. Biomedicines 2021, 9, 81.
- González, M.I.; Martín-Duque, P.; Desco, M.; Salinas, B. Radioactive Labeling of Milk-Derived Exosomes with 99mTc and In Vivo Tracking by SPECT Imaging. Nanomaterials 2020, 10, 1062.
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte‘t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of Sample Collection, Isolation and Analysis Methods in Extracellular Vesicle Research. J. Extracell. Vesicles 2013, 2, 20360.
- Buschmann, D.; Mussack, V.; Byrd, J.B. Separation, Characterization, and Standardization of Extracellular Vesicles for Drug Delivery Applications. Adv. Drug Deliv. Rev. 2021, 174, 348–368.
- Vaswani, K.; Koh, Y.Q.; Almughlliq, F.B.; Peiris, H.N.; Mitchell, M.D. A Method for the Isolation and Enrichment of Purified Bovine Milk Exosomes. Reprod. Biol. 2017, 17, 341–348.
- Samuel, M.; Chisanga, D.; Liem, M.; Keerthikumar, S.; Anand, S.; Ang, C.-S.; Adda, C.G.; Versteegen, E.; Jois, M.; Mathivanan, S. Bovine Milk-Derived Exosomes from Colostrum Are Enriched with Proteins Implicated in Immune Response and Growth. Sci. Rep. 2017, 7, 5933.
- Sukreet, S.; Braga, C.P.; An, T.T.; Adamec, J.; Cui, J.; Trible, B.; Zempleni, J. Isolation of Extracellular Vesicles from Byproducts of Cheesemaking by Tangential Flow Filtration Yields Heterogeneous Fractions of Nanoparticles. J. Dairy Sci. 2021, 104, 9478–9493.
- Raju, D.; Bathini, S.; Badilescu, S.; Ghosh, A.; Packirisamy, M. Microfluidic Platforms for the Isolation and Detection of Exosomes: A Brief Review. Micromachines 2022, 13, 730.
- Wijenayake, S.; Eisha, S.; Tawhidi, Z.; Pitino, M.A.; Steele, M.A.; Fleming, A.S.; McGowan, P.O. Comparison of Methods for Pre-Processing, Exosome Isolation, and RNA Extraction in Unpasteurized Bovine and Human Milk. PLoS ONE 2021, 16, e0257633.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
This entry is offline, you can click here to edit this entry!
