Obesity is a serious health problem because it is rapidly expanding and at the origin of intense morbidity and mortality, especially due to the diabetes that accompanies it.It is not possible to cure it effectively, except with bariatric surgery, which is highly invasive and with many limitations. Trying to understand the mechanism of this efficacy, the studies point towards the correction of the altered control by the CNS of food intake and energy homeostasis. At the biomolecular level, the possibility of a pharmacological therapy based on the modulation of the post-synaptic receptors of glutamate, in particular of the NMDA GluN2A and GluN2B subunits, is foreseen.
- Obesity pathogenesis
- Mechanism of metabolic surgery
- Obesity and diabetes therapy
- NMDA receptors modulation
1. Introduction
Diabetes and obesity have been classified as the third and fourth leading health risk factors, respectively, in the world [1]. Their growing prevalence cannot be countered or even limited, and they are considered to be epidemics. Adiposity-based chronic disease is probably the greatest noninfectious epidemic of the 21st century.
2. Why Is the Spread of Obesity So Rapid?
Role of Epigenetics: Development Programming
The cause of the rapid spread of obesity is partly related to the spread of a lifestyle characterized by a marked reduction in physical activity and a high-calorie diet.
However, the main cause is to be found in genetic adaptations [2], which cannot involve a change in the DNA sequence (because this would require a very long time to manifest). Rather, the change involves epigenetic modifications, a hereditary process of gene expression independent of the classical Mendelian laws [3]. Epigenetics causes organisms to adapt during the first phase of fetal development in a manner that renders them able to cope with changes in the environment [4]. In this phase, called development plasticity, epigenetic modifications are known to be of crucial importance in the development of neural circuits responsible for energy homeostasis and in the integration of autonomic reflexes [5][6].
3. How Can Negative Environmental Conditions Influence The Development of Obesity?
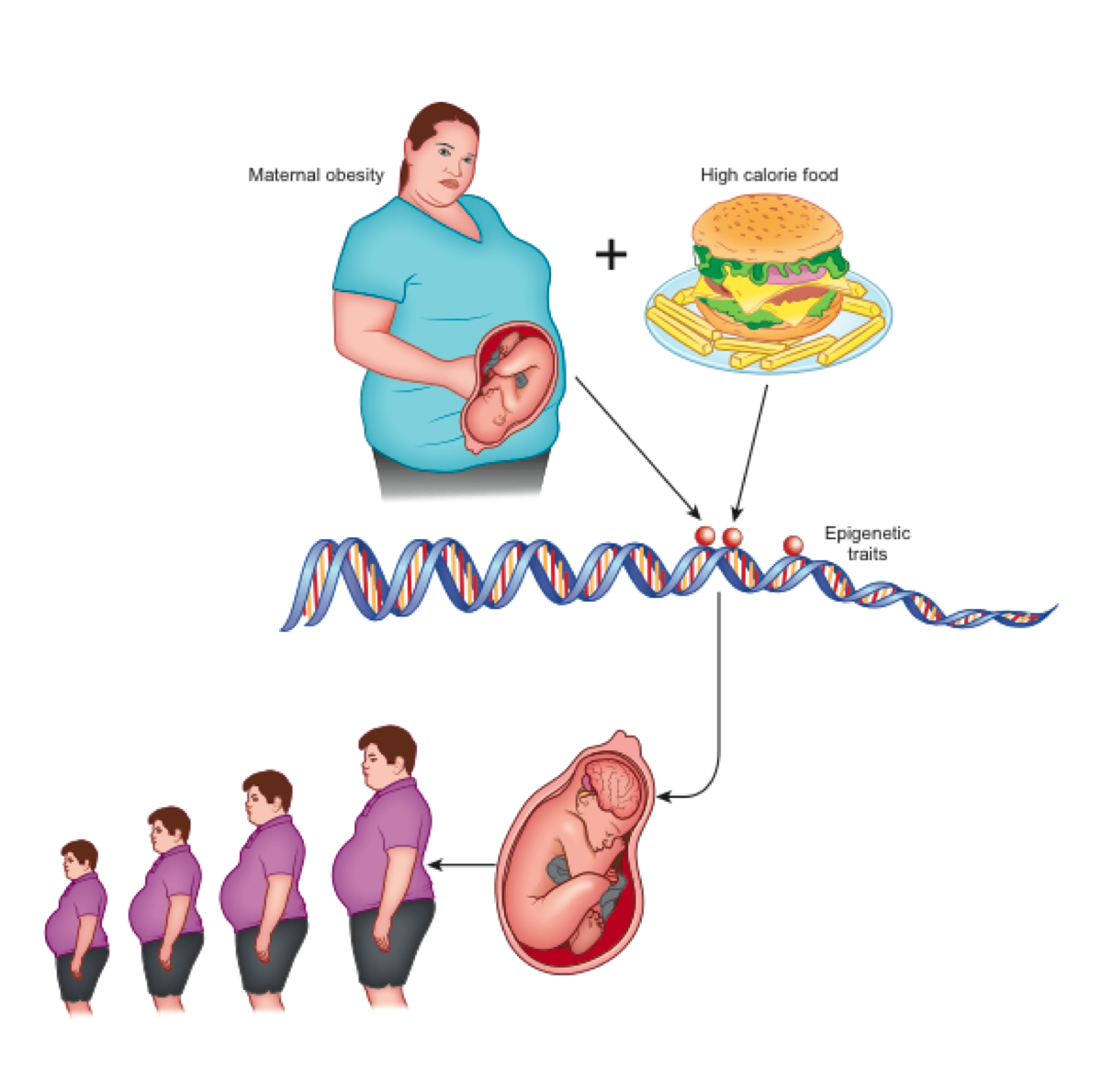
According to the theory of development programming [7], maternal conditions cause adaptive epigenetic changes in the fetus that influence the development of its organs, particularly the brain [4], in view of an adverse post-natal environment [8]. Nutritional excesses are among the most important stimuli of negative fetal programming [9]. In the case of obesity, a metabolic aptitude to caloric accumulation is transferred to the unborn child who, in a subsequent environment with abundant food availability, would be predisposed to the obesogenic phenotype (evolutionary mismatch) [4]. This phenotype is transmitted to subsequent generations even in the absence of further environmental stimuli and, indeed, despite their absence (transgenerational effects) [10] (Figure 1).
Figure 1. Maternal obesity and overeating at the origin of epigenetic changes that lead to neurologic-based generational predisposition to obesity.
In animal models, it has also been shown that a change in synaptic activation (synaptic plasticity) is induced in the reward system [4] followed by an inadequate response to the consumption of highly energetic food in concomitance with the pregnancy period [2][11]. This is due to the epigenetic alteration of the expression of dopamine and opioid-related genes present in the mesocorticolimbic circuits [12].
4. How could the dysfunction of energy homeostasis control be responsible for obesity?
The control of energy homeostasis is performed by various brain sectors that interact with each other [13] (Figure 2).
Figure 2. The main sectors of the central nervous system (CNS) that control the body's energy homeostasis.
4.1 The reward system
The emotional aspects (sensitivity to rewarding stimuli) of non-homeostatic eating behavior (i.e., hedonic food consumption, not activated by energy needs) are determined by the reward system, a grouping of dopaminergic brain structures and neuronal pathways that communicate with each other through the dopamine neurotransmitter and the opioids [14][15]. Chronic consumption of rewarding foods can cause a downregulation of dopamine receptors to attenuate their over-stimulation [16]. Decreased dopamine receptor availability may cause or exacerbate increased food intake as a form of self-medication in which the individual tries to compensate for a reduced reward experience [17].
According to a widely accepted theory called “Reward Deficiency Syndrome” or “Hypodopaminergic Theory” [18], by reducing food intake, withdrawal symptoms appear, such as anxiety, nervousness, dysphoria, and insomnia in both humans [19] and experimental animals [20], and the appetitive and consumer behavior is accentuated [14]. This helps to explain weight loss resistance and why any weight loss induced by calorie restriction recovers very quickly [14].
4.2 The brainstem and the gut–brain axis
The vagal brainstem neurocircuits that regulate food intake and energy homeostasis, through the disorders of their synaptic connections, play an important role in the pathogenesis of obesity and type 2 diabetes mellitus [21]. Enteric hormones stimulate the receptors of a vast network of vagal nerve fibers. Vagal afferent neurons (VAN), located in the nodosum ganglion (NG), innervate the intestine and terminate in the NTS. These afferent vagal fibers integrate gut-derived signals to regulate meal size [21]. From the NTS, impulses start towards other regions of the brain, including the hypothalamic and limbic nuclei, which play a significant role in the processing of neuroendocrine, behavioral, and autonomic functions related to weight control [22] and modulating their functions (such as motivation and pleasure): this is the so-called gut–brain axis [23]. In addition, through the adjacent dorsomedial nucleus (DMV), the NTS connects with the main splanchnic organs that control the metabolism, i.e., the endocrine pancreas and the liver. The deterioration of the synaptic connection with the DMV (synaptic maladaptation) leads to a disordered modulation of the splanchnic organs with their consequent dysfunction (altered production of insulin and glucagon, and impaired hepatic synthesis and release of glucose) [24].
4.3 The hypothalamus
The various nuclei of the hypothalamus play an important role in maintaining energy homeostasis by responding to peripheral hormonal and neural signals, and are activated to control any reduction in adiposity [14]. According to the set point theory, the hypothalamus keeps the quantity of adipose tissue (energy reserve) constant by controlling its utilization and accumulation [14]. According to this theory, in obesity the set point is fixed at a higher level, and this represents another significant obstacle to effective and long-term weight loss . Furthermore, within the hypothalamic lateral, dorsomedial, and peri-fornical regions neurons are present that secrete the orexin neuropeptide, which causes an increase in spontaneous physical activity (SPA) and energy consumption [25]. Other important roles in energy homeostasis are played by the cerebellum and areas of the cortex.
5. Effectiveness of metabolic surgery: which could be the mechanisms?
Bariatric surgery, is currently the most effective therapeutic approach for obesity (consistent and lasting weight loss) and Type 2 DM (average improvement in HbA1c was 2.1% in operated patients compared to 0.3% in patients undergoing intensive medical care) [26]. Metabolic improvements occur within days or weeks following surgery, even before significant weight loss occurs. Therefore, mechanisms are triggered that go beyond simple weight loss and calorie restriction [26].
Surgical procedures sever the branches of the vagus nerve, altering its connection with the brainstem’s dorsal vagal complex (DVC) and other brain areas with which it is linked, in particular those of the reward system [27].
5.1. Changes to feeding
Consequences are: the change in tastes and the apparent aversion to more caloric foods (especially lipids) and the shift towards healthier foods such as fruit and vegetables, in addition to a reduction in the duration and size of meals (even in the presence of weight loss which is usually a powerful stimulus to eat) [14][28][29]. Surgery corrects the dopaminergic dysregulation in the reward system and restores its sensitivity [28][30]. Thus, the peripheral signals are again able to cause satiety and overeating stops [18].
GLP-1 has been evaluated after various bariatric surgeries to determine if it played a role in the dramatic energy-metabolic improvement. It was found that GLP-1 levels increase up to six times after RYGB surgery [31]. Although the topic has been the subject of much debate [32], despite this high increase, GLP-1 cannot be considered the main actor of the improvements because the same effects are obtained in animals deficient for the GLP-1 receptor [33]; the administration of the analogue of GLP1, liraglutide, does not achieve the same effects [33] and when Exenedine-9 (the GLP-1 receptor antagonist) was administered after RYGB, although worsening of glycemic control was observed, there was no return to the initial altered glucose tolerance [34]. It should also be borne in mind that the activity of enteric hormones is predominantly local (paracrine, on vagal fiber receptors) as they are rapidly degraded. Only a limited portion of these hormones also enters the circulation, carrying out their action remotely.
5.2. Change in GLP-1 activity
GLP-1 has been evaluated after various bariatric surgeries to determine if it played a role in the dramatic energy-metabolic improvement. It was found that GLP-1 levels increase up to six times after RYGB surgery [31]. Despite this high increase, GLP-1 cannot be considered the main actor of the improvements because the same effects are obtained in animals deficient for the GLP-1 receptor [34]; the administration of the analogue of GLP1, liraglutide, does not achieve the same effects [33] and when Exenedine-9 (the GLP-1 receptor antagonist) was administered after RYGB, although worsening of glycemic control was observed, there was no return to the initial altered glucose tolerance [34].
5.3. Alteration of vagal afferent neurons
Chronic consumption of a high-fat diet (HFD) causes alterations in the properties of vagal neurons that are sufficient to cause hyperphagia and weight gain, a demonstration that abnormal gut–brain signaling is the triggering factor for obesity [35]. Thus, the high-calorie diet or overfeeding act on two sectors: that of the reward brain by desinsitizing dopamine receptors and causing food addiction and that of alterations in both the function and of the structural integrity of the afferent vagal neurons and of the vagal nuclei of the brainstem by altering the gut-brain-liver axis. These abnormalities regress after RYGB with positive effects on the visceral and gastrointestinal reflexes mediated by the vagus [36].
5.4. Reduced hepatic glucose production (HGP)
It is now certain that the exclusion of the foregut is the main culprit of the benefits of metabolic surgery [37][38]. Diabetes and obesity are characterized by excessive hepatic glucose production. Metabolic surgery, which interrupts (or reduces) the contact between food and the duodenal mucosa, could interfere with afferent and efferent GI-brain connections [39] and determine rapid improvement of the metabolic context in operated patients (regardless of weight loss) through the reduction of HGP [38] (Figure 3).
Figure 3. Nervous control of hepatic glucose production by nutrients in the first part of the intestine, through the brainstem vagal nuclei.
5.5. Accelerated gastric emptying in sleeve gastrectomy (SG)
In SG, which is currently the most practiced type of metabolic surgery (in which the stomach is reduced to about 20% by dissecting it along the greater curvature), the passage of food into the duodenum is preserved but, notwithstanding, it exerts an anti-diabetic effect similar to RYGB which is not due to the restriction of the stomach volume [40]. The anatomical upheaval of the gastric structure by SG, modifying the food gastric processing, inevitably affects the neuro-hormonal functions of the duodenum.
5.6. Modification of the hypothalamic set point
Following bariatric surgery and consequent changes in vagal inputs, this set point could be reset to a normal level, and the defense of excess body fat interrupted [41]. Patients report experiencing less (rather than more) appetite, even in the face of significant weight loss . Furthermore, a resumption of spontaneous physical activity cannot be excluded through the reactivation of the hypothalamic neurons that produce orexin, thus contributing significantly to weight loss [42].
6. Main targets of non-surgical therapeutic approaches to obesity
6.1. Modulation of glutamate receptors
The post-synaptic N-methyl-D-aspartate (NMDAR) receptor, activated by the neurotransmitter glutamate, plays critical roles in the physiological function of the mammalian central nervous system (CNS). Among the receptor's various subunit identities (which confer distinct pharmacological and biophysical properties on the receptor and, consequently, on neuronal processes [43][44]) the subunits GluN1 and GluN2A-GluN2B are involved in the control of nutrition and energy homeostasis [43][45] (Figure 4). Positive allosteric modulators (PAMs) (a group of substances which connect to binding sites different from those of the other agonists of the receptor to modify its response to the stimulus) have been identified that could modulate the activity of these subunits and correct their potential dysfunction at the origin of obesity [46][47].
Figure 4. The NMDA receptors with two of its four subunits: GluN1 and GluN2 and their main binding sites. It acts as a gate on the cell membrane to allow the entry and exit of electrolytes that determine post-synaptic activation (from: Duman, R. (2018). Ketamine and rapid-acting antidepressants: a new era in the battle against depression and suicide. F1000Research, 7).
6.2. Physical methodologies
6.2.1 Electrical stimulation of the vagus nerve
The central role of the intestinal vagus nerve in the origin of obesity (as described above) makes it a valuable target of physical treatment [48]. Percutaneous CT-guided cry-ablation of the posterior vagal trunk causes appetite reduction and weight loss in subjects affected by mild or moderate obesity [49]. A similar effect is obtained by repeated electrical stimulation of the left cervical branch [50].
The ability to selectively modify the plasticity of various brain sectors through afferent vagus stimulation (VNS), which triggers the release of acetylcholine and norepinephrine, represents a remarkable opportunity to treat a variety of brain dysfunctions, including those at the origin of obesity [50].
6.2.2 Brain neuro-modulation
Deep brain stimulation is an invasive but non-damaging neurosurgical procedure which, through electrodes implanted in the brain, sends electrical impulses that reversibly modify the activity of nerve cells and is capable of positively influencing eating behavior in obesity [51][52].
Non-invasive brain stimulation (or transcranial magnetic stimulation is a technique in which the electric current of a coil placed on the skull generates a magnetic field that induces a parallel intracranial current [53]. Activation of the cortex is provoked, obtaining an improvement of various forms of addiction [54] and obesity [55]—understood as a food addiction condition—thus reducing food intake [56]. The main target of this treatment is the dorsolateral prefrontal cortex, responsible for self-control in food intake [53][57]. Beneficial effects have been obtained in various alterations of eating behavior, including binge eating [53]. Some limitations still need to be resolved, such as the need for repeated applications and the difficulty in accurately detecting targets, but this could represent a promising therapeutic path [53][56].
7. Conclusions
Obesity has reached epidemic proportions. The mechanisms can be found in the dysfunction of various sectors of the CNS that are responsible for energy homeostasis and are conditioned by epigenetic alterations. At present, the most effective and long-lasting therapy for obesity and related diabetes is metabolic surgery (which, however, is not universally practicable and is not devoid of side effects). Researchers' efforts should be directed towards further clarification of the neuro-hormonal bases of obesity and of the therapeutic mechanisms of surgery, aiming to identify drugs that will be active on the receptors of the various neurotransmitters involved, and modulating the activity of the vagal nervous system on the control of energy homeostasis and gastrointestinal functions. Particular attention may be warranted for the allosteric activation of the GluN2A and GluN2B subunits of NMDA receptor. The final purpose would be to achieve the same surgical effects by means of non-surgical methods [58].
This entry is adapted from the peer-reviewed paper 10.3390/gidisord2040039
References
- Lim SS, Vos T, Flaxman, AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2224–60
- Reynolds CM, Segovia SA, Vickers MH. Experimental models of maternal obesity and neuroendocrine programming of metabolic disorders in offspring. Front Endocrinol (Lausanne) 2017;8:245-54
- Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, Leibel RL. Obesity pathogenesis: an endocrine society scientific statement. Endocrine reviews2017;38(4):267–96
- Montalvo-Martínez L, Maldonado-Ruiz R, Cárdenas-Tueme M, Reséndez-Pérez D, Camacho A. Maternal overnutrition programs central inflammation and addiction-like behavior in offspring. Biomed Res Int 2018;8061389
- Levin BE. Interaction of perinatal and pre-pubertal factors with genetic predisposition in the development of neural pathways involved in the regulation of energy homeostasis. Brain Res 2010;1350:10‐7
- Bhagat R, Fortna SR, Browning KN. Exposure to a high fat diet during the perinatal period alters vagal motoneurone excitability, even in the absence of obesity. J Physiol 2015;593(1):285-303
- Barker D J. The fetal and infant origins of adult disease. BMJ 1990;301:1111-23
- Vickers MH. Developmental programming and transgenerational transmission of obesity. Ann Nutr Metab 2014;64,Suppl 1:26-34
- Franzago M, Fraticelli F, Stuppia L, Vitacolonna E. Nutrigenetics, epigenetics and gestational diabetes: consequences in mother and child. Epigenetics 2019;14(3):215-35
- Rando OJ, Simmons RA. I'm eating for two: parental dietary effects on offspring metabolism. Cell 2015;161(1):93-105
- Walker CD. Development, brain plasticity and reward: early high-fat diet exposure confers vulnerability to obesity-view from the chair. Int J Obes Suppl 2012;2(Suppl 2):S3–6
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 2010;151(10):4756-64
- Smith JK. Exercise, obesity and CNS control of metabolic homeostasis: a review. Front Physiol 2018;9:574-615
- Behary P, Miras AD. Brain responses to food and weight loss. Exp Physiol 2014;99(9):1121-7
- Sasaki T. Neural and molecular mechanisms involved in controlling the quality of feeding behavior: diet selection and feeding patterns. Nutrients 2017;9(10):1151-62
- Adams RC, Sedgmond J, Maizey L, Chambers CD, Lawrence NS. Food Addiction: implications for the diagnosis and treatment of overeating. Nutrients 2019;11(9):2086-98
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet 2001;357:354–7
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond, B, Biol Sci 2008;363:3191-200
- Kalon E, Hong JY, Tobin C, Schulte T. Psychological and neurobiological correlates of food addiction. Int Rev Neurobiol 2016;129:85-110
- Albaugh VL, Flynn CR, Tamboli RA, Abumrad NN. Recent advances in metabolic and bariatric surgery. F1000Res 2016; vol 5:F1000 Faculty Rev-978
- Bove C, Travagli RA. The Vagus Nerve. In Encyclopedia of Gastroenterology, 2nd ed. Academic Press, 2020, Oxford, UK, pp. 676-82
- Bülbül M, Travagli RA. Novel transmitters in brain stem vagal neurocircuitry: new players on the pitch. Am J Physiol Gastrointest Liver Physiol 2018;315(1):G20-6
- Liddle RA. Neuropods. Cell Mol Gastroenterol Hepatol 2019;7(4):739-47
- Blasi C. The role of the vagal nucleus tractus solitarius in the therapeutic effects of obesity surgery and other interventional therapies on type 2 diabetes. Obes Surg 2016:26(12):3045-57
- Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol Regul Integr Comp Physiol 2008;294(3):R699-R710
- Cornejo-Pareja I, Clemente-Postigo M, Tinahones FJ. Metabolic and endocrine consequences of bariatric surgery. Front Endocrinol (Lausanne) 2019;10:626-717
- Peters JH, Gallaher ZR, Ryu V, Czaja K. Withdrawal and restoration of central vagal afferents within the dorsal vagal complex following subdiaphragmatic vagotomy. J Comp Neurol 2013; 52(15):3584-99
- Thanos PK, Michaelides M, Subrize M, Miller ML, Bellezza R, Cooney RN, Leggio L, Wang GJ, Rogers AM, Volkow ND, Hajnal A. Roux-en-Y gastric bypass alters brain activity in regions that underlie reward and taste perception. PloS one2015;10(6):e0125570
- Kittrell H, Graber W, Mariani E, Czaja K, Hajnal A, Di Lorenzo PM. Taste and odor preferences following Roux-en-Y surgery in humans. PLoS ONE 2018;13(7):e0199508
- Eickhoff H. Central modulation of energy homeostasis and cognitive performance after bariatric surgery. Adv Neurobiol 2017;19:213-36
- Elliott JA, Reynolds JV, le Roux CW, Docherty NG. Physiology, pathophysiology and therapeutic implications of enteroendocrine control of food intake. Expert Rev Endocrinol Metab 2016;11(6):475-99
- Hutch CR, Sandoval D. The role of GLP-1 in the metabolic success of bariatric surgery. Endocrinology 2017;158(12):4139-51
- Ye J, Hao Z, Mumphrey MB, Townsend RL, Patterson LM, Stylopoulos N, Münzberg H, Morrison CD, Drucker DJ, Berthoud HR. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. American journal of physiology. Regulatory, integrative and comparative physiology 2014;306(5):R352–R62
- El Khoury L, Chouillard E, Chahine E, Saikaly E, Debs T, Kassir R. Metabolic surgery and diabesity: a systematic review. Obes Surg 2018;28(7):2069-77
- Kim JS, Kirkland RA, Lee SH, Cawthon CR, Rzepka KW, Minaya DM, de Lartigue G, Czaja K., de La Serre CB. Gut microbiota composition modulates inflammation and structure of the vagal afferent pathway. Physiology & behavior 2020; 225:113082
- Browning KN, Fortna SR, Hajnal A. Roux-en-Y gastric bypass reverses the effects of diet-induced obesity to inhibit the responsiveness of central vagal motoneurones. J Physiol 2013; 591(9):2357-72
- Laessle C, Nenova G. Duodenal exclusion but not sleeve gastrectomy preserves insulin secretion, making it the more effective metabolic procedure. Obes Surg 2018;28(5):1408-16
- Rubino F. Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care 2008;31(Suppl 2):S290–6
- Scarlett JM, Schwartz MW. Gut-brain mechanisms controlling glucose homeostasis. F1000Prime Rep 2015;7:12-20
- Chambers AP, Smith EP, Begg DP, Grayson BE, Sisley S, Greer T, Sorrell J, Lemmen L, LaSance K, Woods SC, Seeley RJ, D'Alessio DA, Sandoval DA. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. American journal of physiology. Endocrinology and metabolism 2014; 306(4):E424–E32
- Raghow R. Gut-brain crosstalk regulates craving for fatty food. World J Diabetes 2017;8(12): 484-8
- Chieffi S, Carotenuto M, Monda V, Valenzano A, Villano I, Precenzano F, Tafuri D, Salerno M, Filippi N, Nuccio F, Ruberto M, De Luca V, Cipolloni L, Cibelli G, Mollica MP, Iacono D, Nigro E, Monda M, Messina G, Messina A. Orexin system: the key for a healthy life. Front Physiol 2017;8:357-66
- Üner A, Gonçalves GH, Li W, Porceban M, Caron N, Schönke M, Delpire E, Sakimura K, Bjørbæk C. The role of GluN2A and GluN2B NMDA receptor subunits in AgRP and POMC neurons on body weight and glucose homeostasis. Molecular metabolism 2015;4(10):678–91
- Wu Q, Zheng R, Srisai D, McKnight GS, Palmiter RD. NR2B subunit of the NMDA glutamate receptor regulates appetite in the parabrachial nucleus. Proc Natl Acad Sci USA 2013;110:14765–70
- Minaya DM, Larson RW, Podlasz P, Czaja K. Glutamate-dependent regulation of food intake is altered with age through changes in NMDA receptor phenotypes on vagal afferent neurons. Physiol Behav 2018;189:26-31
- Goldsmith PJ. NMDAR PAMs: multiple chemotypes for multiple binding sites. Curr Top Med Chem 2019;19(24):2239-53
- Hackos DH, Hanson JE. Diverse modes of NMDA receptor positive allosteric modulation: Mechanisms and consequences. Neuropharmacology 2017;112:34–45
- Johannessen H, Revesz D, Kodama Y, Cassie N, Skibicka KP, Barrett P, Dickson S, Holst J, Rehfeld J, van der Plasse G, Adan R, Kulseng B, Ben-Menachem E, Zhao CM, Chen D. Vagal blocking for obesity control: a possible mechanism-of-action. Obesity surgery 2017;27(1):177–85
- Prologo JD. Percutaneous CT-guided cryovagotomy. Tech Vasc Interv Radiol 2020;23(1):100660.
- Hays SA, Rennaker RL, Kilgard MP. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res 2013;207:275-99
- Dalton B, Campbell IC, Schmidt U. Neuromodulation and neurofeedback treatments in eating disorders and obesity. Curr Opin Psychiatry 2017;30(6):458-73
- Whiting AC, Oh MY, Whiting DM. Deep brain stimulation for appetite disorders: a review. Neurosurg Focus 2018;45(2):E9
- Jáuregui-Lobera I, Martínez-Quiñones JV. Neuromodulation in eating disorders and obesity: a promising way of treatment? Neuropsychiatr Dis Treat 2018;14:2817-35
- Gorelick DA, Zangen A, George MS. Transcranial magnetic stimulation in the treatment of substance addiction. Ann NY Acad Sci 2014;1327:79-93
- Ljubisavljevic M, Maxood K, Bjekic J, Oommen J, Nagelkerke N. Long-term effects of repeated prefrontal cortex transcranial direct current stimulation (tDCS) on food craving in normal and overweight young adults. Brain Stimul 2016;9(6):826-33
- Lee DJ, Elias GJB, Lozano AM. Neuromodulation for the treatment of eating disorders and obesity. Ther Adv Psychopharmacol 2018;8(2):73-92
- Hall PA, Vincent CM, Burhan AM. Non-invasive brain stimulation for food cravings, consumption, and disorders of eating: A review of methods, findings and controversies. Appetite 2018;124:78-88
- van der Klaauw AA. Neuropeptides in Obesity and Metabolic Disease. Clin Chem 2018;64(1):173-82