Insulin resistance is a critical pathophysiological process in the onset and advancement of type 2 diabetes mellitus. It is well-recognized that alterations in the metabolism of lipids and aberrant fat buildup effectively trigger the development of resistance to insulin. Adjusting one’s eating habits and managing weight appropriately are crucial for treating, controlling, and reducing the risk of T2DM because obesity and a lack of physical exercise are the primary factors responsible for the worldwide rise in T2DM. Omega-3 fatty acid is one of the polyunsaturated fatty acids (PUFA) that include long-chain omega-3 fatty acids such as eicosapentaenoic acid and docosahexaenoic acid, commonly found in fish oils.
1. Introduction
Insulin resistance (IR) is considered the reduced responsiveness of peripheral tissues to insulin, and it was observed that IR consequences take place several years before type 2 diabetes mellitus (T2DM) [
1]. T2DM raises possibilities for retinopathy, renal impairment, cardiovascular events, and amputation of the lower limb and thus becomes a primary cause of mortality [
2]. Studies showed that abnormalities in insulin generation and/or function lead to a deterioration of glycemic control and culminate in the development of T2DM, which tends to result in dyslipidemia [
3]. Since obesity and inadequate physical activity are the leading causes of the resultant increase in T2DM globally, adjusting one’s food-taking behavior and appropriate weight management is fundamental for curing, limiting, and reducing the risk of T2DM [
4]. Hypertension, T2DM, and obesity are all factors in metabolic syndrome (MetS). The underlying causes of MetS and obesity include inflammation and disturbance of adipose tissue functioning [
5]. Adipokines, bioactive peptides, and lipids secreted by adipose tissue control inflammation, insulin sensitivity, cardiovascular function, and adipose tissue function [
6]. Adipose tissue secretes large amounts of interleukin-10 (IL-10) and other anti-inflammatory mediators in physically active persons [
7].
On the other hand, in obese people, adipose tissue secretes a lot of pro-inflammatory adipokines, such as tumor necrosis factor (TNF alpha), monocyte chemoattractant protein 1 (MCP-1), and IL-1beta [
8]. The incidence of T2DM can also be minimized if saturated fat is replaced with unsaturated fat [
9]. Fatty acids serve as a source of energy, a vital component of biological membranes, and interact with various receptors and transcription factors, besides acting as precursors to paracrine mediators such as prostaglandins [
10].
Glucose homeostasis is adversely affected by IR in skeletal muscle and the liver. Around 80% of postprandial glucose disposal occurs in skeletal muscle. Insulin activity and glucose homeostasis are greatly affected by the decreased glucose uptake that occurs in IR, which is predominantly caused by the inappropriate control of glucose transporter-4 (GLUT4) [
11]. In the insulin-resistant state, the liver’s ability to regulate gluconeogenesis and glycogenolysis is hampered, compromising the capability to regulate glucose production. Additionally, the poor regulation of lipolysis in the white adipose tissue (WAT) is a factor in the hyperlipidemia found in insulin-resistant conditions [
12].
Omega-3 fatty acid is one of the polyunsaturated fatty acids (PUFA) that include long-chain omega-3 fatty acids such as eicosapentaenoic acid and docosahexaenoic acid. These are commonly found in fish, and among the plant oils that contain alpha-linolenic acid are flaxseed, rapeseed, and canola [
13]. The American Diabetes Association recommends a Mediterranean-style diet without supplementation that is rich in polyunsaturated, long-chain omega-3 fatty acids and alpha-linolenic acid [
14,
15]. Patients in the UK with T2DM are also encouraged to eat oily fish without supplementation [
16]. PUFA should be substituted for saturated fats to reduce total and saturated fat intake and avoid diabetes [
17]. Long-standing concerns about long-chain omega-3 fatty acids’ impact on diabetes management have been supported by experimental investigations that found significant increases in fasting glucose following omega-3 fatty acid supplementation and foods rich in PUFA and omega-3 fatty acid [
18]. High quantities of methylmercury and polychlorinated biphenyl have been found in seafood and fish oil supplements; in addition to this, these levels impair insulin signaling and cause fasting glucose to increase in animal models [
19,
20].
Cellular explanations to explain the connection between inflammation and IR include mitochondrial dysfunction, endoplasmic reticulum (ER) stress, and oxidative stress. The mitochondria and the ER are susceptible to stress in situations involving persistent malnutrition and positive body energy balance because of excess nutrients and metabolic requirements [
21]. The stress experienced by mitochondria and ER triggers the unfolded protein response (UPR) that, in turn, stimulates the main inflammatory processes and hinders insulin action [
22]. Nutritional supplements and/or biologically active substances with anti-inflammatory effects may be crucial in preventing and treating insulin resistance [
23]. Omega-3 polyunsaturated fatty acids (PUFA), a nutrient, have been demonstrated to have bioactive qualities associated with their recognized anti-inflammatory benefits. Investigating the molecular and cellular mechanisms of IR is a crucial area of research for the evolution of precautionary therapeutics for metabolic conditions that advance T2DM and its concurrent illnesses. Omega-3 PUFA may affect metabolic activity and the treatment of insulin resistance in individuals, but further study is required to fully comprehend how they affect inflammatory processes and cell metabolism [
24].
2. Polyunsaturated Fatty Acid (PUFA)
PUFAs are fatty acids containing 18–24 carbons and more than 2 double bonds. A healthy body requires omega-3 and omega-6 PUFAs, which cannot be produced by the human body and should be acquired through organic foods such as walnut, flaxseed, and fish [
25] (
Figure 1). Many studies have revealed that omega-3 PUFAs such as eicosapentaenoic (EPA) and docosahexaenoic (DHA) can help with metabolic issues such as IR, obesity, atherosclerosis, and chronic inflammation [
26]. DHA can significantly increase the number of mitochondria in muscles, lower stress before birth and oxidative damage to mitochondrial DNA, and enhance mitochondrial activity in neurodegenerative conditions [
27]. The exact molecular processes by which omega-3 PUFAs control mitochondrial activity to defend against IR are still unknown.
Figure 1. Diagram showing dietary sources of omega-3 fatty acids. This figure has been drawn utilizing the premium version of BioRender with the license number AJ25A0VPGX. Image Credit: Susmita Sinha.
3. Insulin Signaling
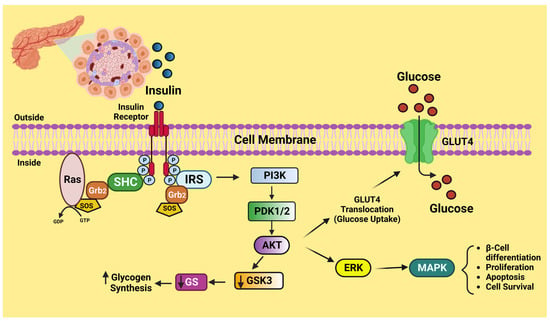
Insulin, a peptide hormone released by the pancreatic beta-cells in response to elevated blood glucose, is the critical regulator of human carbohydrate metabolism. Insulin also prevents lipolysis in adipose tissue, which decreases the release of free fatty acids from adipose tissue (FFA) and thereby lowers blood FFA levels [
28]. The insulin-sensitive tissues are muscle, adipocytes, and the liver, which undergo a cascade of intracellular signaling processes as a consequence of insulin attaching to its tetrameric receptor expressed on the cell membrane. Once insulin binds to its receptor, structural changes take place, causing some tyrosine residues to become autophosphorylated. The phosphatidylinositol 3-kinase and protein kinase B (PI3K)-AKT is triggered when the activated kinase phosphorylates tyrosine residues on insulin receptor substrates (IRS). In order to mediate insulin-induced absorption of glucose and decrease gluconeogenesis, the PI3K-AKT path is acknowledged for playing a crucial role [
29] (
Figure 2). The serine kinase and c-Jun N-terminal kinase (JNK) counteract the actions of PI3K-AKT. In addition, serine kinase promotes the pro-inflammatory intracellular signaling paths and functions as a negative regulator of insulin signaling [
30]. When there is inflammation, the molecular signals from an inflammatory situation disrupt insulin signaling, provoking a decrease in the cell’s molecular sensitivity to insulin.
Figure 2. Schematic diagram of insulin signaling. This figure has been drawn utilizing the premium version of BioRender with the license number TW25A0NH90. Image Credit: Susmita Sinha.
4. The Role of Omega-3 PUFA in Insulin Resistance
Omega-3 and omega-6 polyunsaturated fatty acids (PUFAs; 3 and 6 PUFAs) are essential for human health because they serve as metabolic precursors of eicosanoids, a class of signaling molecules that are essential for controlling a body’s inflammation. The omega-3 PUFA are primarily acquired from fish but have recently been found in enhanced foods such as bread and dairy products [
57]. Since humans are unable to produce any of the omega-3 or omega-6 PUFAs, they both constitute essential nutritional ingredients. Omega-3 PUFA comprises alpha-linolenic acid (ALA) and longer-chain fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which can be extracted from seafood. Because humans lack the natural desaturase enzymes necessary for its synthesis, ALA, an essential fatty acid, cannot be produced by the body and must be obtained through diet. ALA is then transformed into the long-chain omega-3 PUFA, EPA, or DHA, in the human body [
58].
The structural components of omega-3 PUFA are necessary for forming cell walls and aid in maintaining membrane flexibility. Enhanced cell-to-cell communication and appropriate homeostasis are both supported by increasing membrane fluidity. Through its impact on cell membrane structure and/or gene expression modulation via influencing transcription factors associated with energy supply and cell cycle, omega-3 PUFA may help minimize metabolic diseases. Additionally, it has been established that the primary benefit of omega-3 PUFAs is due to their capacity to decrease inflammation, which is an essential characteristic of obesity and related metabolic diseases [
59].
Numerous studies have shown that omega-3 PUFA can stop or reverse changes in the composition or function of the mitochondria in skeletal muscle. Animals fed a high-fat diet (60% fat) with fish oil (3.4% kcal from n-3 PUFAs) for 10 weeks showed increased expression of the transcriptional factors of mitochondrial biogenesis, including nuclear respiratory factor-1 (NRF1) and peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1a) [
60]. It has been demonstrated that a diet high in fat (200 g fat/kg), including menhaden (fish) oil causes an increase in mitochondrial carnitine palmitoyl transferase 1 (CPT-1) in skeletal muscle and the hearts of rats [
35]. Since CPT-I makes it easier to transport acyl groups into the mitochondria, it serves as the primary control point for beta-oxidation. The expression of CPT-I is governed by peroxisome proliferator-activated receptors (PPARs) and the 5′-AMP-activated protein kinase (AMPK) [
61]. The activation of AMPK by EPA (200 mumol/L for 24 h) in primary cultured rat fat cells and the skeletal muscle of high fat-fed rats supplemented with 10% v/w omega-3 PUFA (as fish oil) for six weeks both led to a noted rise in mitochondrial CPT-1 expression and fatty acid oxidation [
35]. An essential factor in preventing the establishment of insulin resistance is improved fatty acid consumption, which is likely to reduce excessive lipid buildup and lipotoxicity [
62].
Higher expression of uncoupling protein 3 (UCP-3) mRNA in skeletal muscle and increased expression of peroxisomal acyl-CoA oxidase (PACO) in the liver, skeletal muscle, and heart is another explanation suggested to explain the effects of omega-3 PUFA on adipose tissue. A recent study found that feeding obese mice a high-fat diet rich in fish oil (60 percent of calories should come from fat for 12 weeks) enhanced their mice’s glucose tolerance and insulin sensitivity [
63]. Reduced adipose tissue dysfunction and inhibition in high-fat-feeding-induced ER stress were linked to this increased insulin sensitivity [
64]. The inhibition of ER stress by omega-3 PUFA in adipocytes has been attributed to AMPK activation [
35].
Nucleotide-binding and oligomerization domain-like receptor, leucine-rich repeat, and pyrin domain-containing 3 (NLRP3) inflammasome have been proposed to be influenced by omega-3 PUFA in obesity and obesity-related metabolic disorders, such as insulin resistance [
65]. Exposure to pathogens and host danger signals, inflammasomes, and multiprotein complexes mediate the immune system’s first line of defense by activating caspase-1 and inducing the release of IL-1 and IL-18 [
66]. According to certain theories, NLRP3 often detects cellular homeostasis disturbances such as redox status shifts or ER stress [
67]. However, the exact process by which NLRP3 becomes active is still not entirely known. ROS generation and mitochondrial damage can result from a substantial mitochondrial Ca
2+ influx. Consequently, Ca
2+ fluxes may be the intermediary process bridging ER stress, inflammation, and mitochondrial dysfunction paths in the progression of resistance to insulin, and omega-3 PUFA may control this process [
68].
This entry is adapted from the peer-reviewed paper 10.3390/life13061322