Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Applied
The lithium–sulfur (Li-S) battery is considered to be one of the attractive candidates for breaking the limit of specific energy of lithium-ion batteries and has the potential to conquer the related energy storage market due to its advantages of low-cost, high-energy density, high theoretical specific energy, and environmental friendliness issues.
- lithium–sulfur batteries
- low temperatures
- anode
1. Introduction
In the context of the green and environmental protection society, the demand for rechargeable batteries is ever increasing, and the application range is also gradually becoming wider. Meanwhile, it is a significant challenge to break the theoretical bottleneck for commercial lithium-ion batteries (LIBs), consisting of a low-capacity anode and cathode.
Moreover, with the in-depth research of Goodenough et al. on lithium–sulfur (Li-S) batteries, such as the invention and development of key cathode materials for lithium-ion batteries, the potential development of lithium-ion batteries is being limited [1,2]. Li-S batteries have been widely considered because of their higher theoretical energy density, stronger environmental protection ability and lower cost, features with great promise when compared to alternative LIBs [3,4]. Unlike LIBs, working with the ion insertion mechanism [5,6], Li-S batteries are mainly based on the conversion reaction of active materials [7]. The active materials of the positive and negative electrodes are elemental sulfur and lithium metal, respectively, making the Li-S battery a high energy density, high performance, and low-cost sulfur cathode [8,9,10]. Therefore, Li-S batteries have an outstanding contribution to solving the problem of the subsequent development of the battery industry.
Unfortunately, Li-S batteries still face many challenges, especially at low temperatures. Li-S batteries, at low temperatures, suffer from the slow kinetics of cathode and anode reactions, which in turn leads to low capacity and poor cycle performance [11]. In addition, the shuttle effect of soluble lithium polysulfides (LiPSs, Li2Sx, 2 < x ≤ 8), the insulation of S and insoluble sulfide (Li2S2 and Li2S) [12,13], the drastic volume change during charge and discharge, and the inherent dendrite growth of the lithium metal anode play a key role in the electrochemical performance [14].
In recent years, Li-S batteries have attracted extensive attention, but this is mainly focused on their performance at room temperature, including the idea of an interlaminar structure [15], designing novel electrode materials with enhanced electrochemical activity and stability [16,17], and optimizing the electrolyte composition and structure to improve ion transport and reduce the formation of lithium polysulfide [18]. However, there are still few systematic discussions on the operation of Li-S batteries at low temperatures.
2. The Underlying Mechanisms of Li-S Batteries at Low Temperature
The Li-S batteries are composed of a lithium anode, a sulfur cathode, an electrolyte, and a separator, suffering from the dilemma of reduced efficiency or even deactivation when they operate at low temperature. Specifically, the main failure mechanisms of Li-S batteries at low temperature include (i) a high Li ion desolvation energy barrier; (ii) uncontrolled nucleation and deposition of lithium; (iii) LiPSs cluster aggregation; and (iv) cathode passivation caused by Li2S film deposition (Figure 1) [22].
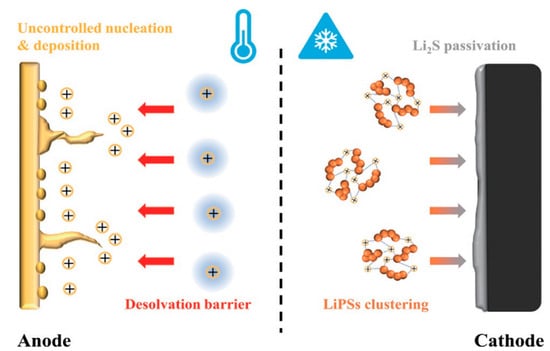
Figure 1. Illustration of Li-S battery failure mechanisms at low temperature.
The high desolvation energy barrier of the Li ions at low temperature was determined to be the key factor leading to the failure of the battery. When the Li ions are bonded with the solvent molecule, the energy barrier for desolvation substantially increases at the electrochemical interface and further weakens local charge transfer capability [23]. To this end, the design strategies of solid electrolyte interphase (SEI) should be reconsidered to promote the dissociation of solvent molecules from the primary dissolved sheath of Li ions [24], which is favorable for the in-depth investigation of the high lithium ion dissolving energy barrier and would further enhance the performance of the lithium metal anode at low temperature.
The uncontrolled nucleation and deposition of lithium have an extremely negative effect on cycle performance. As the temperature decreases, the large-size deposited Li will evolve into dead lithium with an extensive surface, significantly reducing the Coulomb efficiency [25]. Accordingly, it is of great importance to develop effective strategies to mitigate lithium deposition and enhance the activity for Li-S batteries [26].
At low temperature, the low-discharge platform of Li-S batteries is firmly affected by the behavior of Li2S4 clusters [27]. In other words, the formation of Li2S4 clusters inhibit its conversion to Li2S, eventually leading to slow electrochemical reaction kinetics and capacity loss to the secondary voltage platform [28].
Regarding the cathode passivation by the film-like Li2S deposition, the nucleation size of Li2S shrinks with the decreased temperature, which leads to the prepassivation of the electrode surface and disappearance of three-phase interface of the active material, electronic conductor, and ionic conductor (electrolyte), thus terminating the electrochemical reaction. As a result, adjusting the deposition morphology of Li2S is of great significance to improve the capacity and reversibility of the positive electrode at low temperatures [29,30,31,32].
In fact, while the processes that limit the performance of low-temperature Li-S batteries are myriad, they all tend to have the same effect: capacity loss. Under a given voltage window, the reversible capacity of Li-S batteries is decreased with the decrease in temperature. For instance, below −20 °C, the reversible capacity of Li-S batteries is less than 25% of the primary capacity. After raising the temperature again to room temperature, the reversible capacity can be recovered as well. On the other hand, due to the irreversible deposition of lithium metal on the anode surface, capacity loss can also occur when the cell is charged at low temperature [33]. Both capacity degradation mechanisms for Li-S batteries at freezing temperature are mainly attributed to its increased internal resistance, which is caused by different physicochemical processes [34].
Optimizing the cell configurations can improve battery performance and address corresponding issues, which can be approached from various angles, including electrode and electrolyte optimization, and separator/interlayer design. The use of various 3D current collectors, such as porous current collectors, sandwich-type current collectors, and multilayered current collectors, can avoid the cracking. The utilization of binders on the cathode material can also enhance the contact performance between the coating and the electrode, and specific binders can improve specific properties of Li-S batteries. Zhou et al. introduced a binder, ammonium polyphosphate (APP), which not only facilitates the adhesion of electrode coatings but also utilizes its strong affinity with lithium polysulfides to hinder the diffusion and shuttle of polysulfide anions [35]. In terms of electrolyte, incorporating new types of electrolytes and using appropriate solvents and salts are commonly employed methods to improve its electrochemical performance. The addition of redox mediators (RMs) in the electrolyte is a highly promising approach for optimizing Li-S battery configurations, as RMs can regulate the oxidation reactions of sulfur [36]. Regarding the optimization of separators/interlayers, it can be categorized into three functions: (1) dealing with polysulfide diffusion; (2) improving the electrical conductivity for complete sulfur utilization; and (3) enhancing the kinetics of the conversion reactions of sulfur species. They, respectively, correspond to the key issues of polysulfide dissolution and the Shuttle effect, the insulating nature of sulfur species, and the poor kinetics in the scissoring of S-S bonding for Li-S batteries [37].
3. Anode
Since the theoretical specific capacity of sulfur (1673 mAh/g) is much inferior to lithium (3860 mAh/g), the initial energy density is determined by the mass of sulfur in the whole system. Moreover, the instability at the surface and the bulk of the lithium anode during cycling seriously affect the capacity retention and cycle life [38]. According to recent progress, Li-S batteries are suffering from the following challenges and difficulties: (1) the Shuttle effect of Li ions [39]; (2) the uneven deposition of intermediate-term for SEI [40]; and (3) the serious dendrites and self-discharge of the lithium metal anode [4]. Among these factors, the serious dendrites of the lithium metal anode have the greatest impact on safety and stability [41]. The formation of lithium dendrites in lithium-ion batteries is attributed to the repeated deposition and stripping of lithium metal on the surface of electrodes during the charging and discharging process. Pore formation and surface defects can occur as a result of this process, leading to a high local potential that promotes the growth of dendrites [42]. Other factors such as temperature, current density, composition and concentration of electrolytes, electrode materials and structures, and the charging/discharging states of the battery can also contribute to dendrite formation. Lithium dendrites lead to serious safety issues, as they can cause short circuits, puncture the separator, and initiate uncontrolled reactions [43]. In order to overcome these difficulties and obtain stable lithium metal anodes, numerous strategies such as regulating the electrolyte, fabricating an artificial SEI layer, modifying the 3D current collector hosts and/or the lithophilic site, and exploring alternative anode materials have been employed [44]. However, research on Li-S batteries operating at low temperatures is still scarce.
3.1. Exploration of Substitution and Improvement for Anode Materials
3.1.1. Graphite
Graphite, with its flat intercalation potential, good capacity, and long cycle life, can effectively inhibit volume expansion and dendrite formation in lithium metal anodes. However, the related intrinsic drawbacks, such as poor Li diffusion kinetics in the interlayers, low intercalation potential, and relatively large interfacial resistance [45,46], usually result in the Li plating on graphite and restricting extensive applications in low-temperature conditions [45,46,47]. Due to the catalytic effect of the metal during the Li ions desolvation process, when mixed with 1 wt% of metal nanoparticles (Cu, Al, Sn), the light graphite oxide was able to deliver 30% of the theoretical capacity at −30 °C and 0.2 °C [48,49]. In comparison with the original graphite oxide electrode, the graphite oxide with coated and dispersed Sn provided a capacity of 152 and 94 m Ah g−1, respectively, at −30 °C. It was demonstrated that the low-temperature performance of graphite and LTO(Li4Ti5O12) anodes can be effectively improved by using copper nanoparticles on Super-P (Cu/Super-P) as a conductive additive [50,51]. Coating graphite with Al2O3 may also prevent Li deposition, improving its cryogenic performance [52].
The mild oxidation of graphite is expected to solve the problem of reducing the lithium overpotential of the graphite anode. Cao [53] and Wu et al. [54] innovatively utilized the heat treatment and concentrated nitric acid solution to lightly oxidize graphite, which exhibits better cycling performance at low temperatures. The reduced particle size and number of terminal unsaturated carbon atoms, forming nanoscale voids and channels as well as the formation of chemically bonded SEI, was attributed to this mild oxidation treatment [47].
In addition, particle size reduction and structure modification can also improve the low-temperature performance of graphite [55,56]. The combination of thin graphite sheets with through-holes (porous graphite nanosheets, PGNs) and carbon nanotubes (CNTs) significantly shortens the diffusion path. The dominant mesopores and micropores in PGN-CNT anodes facilitate Li ion transport, resulting in a superior rate and performance at low temperatures (Figure 2a,b). After 500 cycles, the capacity retention was maintained at 90% for 0.75 m LiTFSI 1,3-dioxane (DIOX) electrolyte, and the reversible capacity was above 300 mAhg−1 at 0.1 °C and −20 °C. Ionic liquids were used to perform microwave exfoliation on expanded graphite and synthesized multilayer crystalline graphene (GRAL) (Figure 2c) [57]. The high surface area allows efficient electrochemical reactions, as evidenced by the 3–4 times greater capacity of GRAL compared to commercial graphite at −30 °C (Figure 2d). Moreover, oxided mesocarbon microbeads and expanded MCMB were prepared as well. The expanded MCMB with increased interlayer distance delivered 130 and 100 m Ah g−1 at −10 and −40 °C, respectively [58].
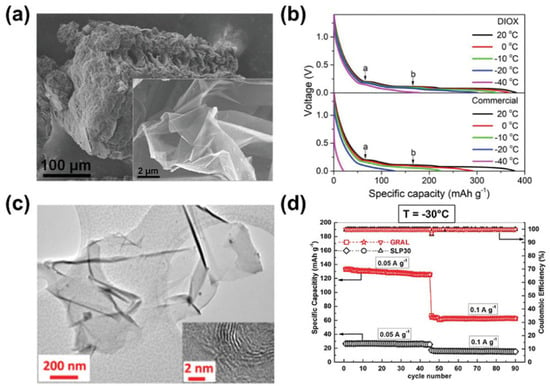
Figure 2. (a) Scanning electron microscopy (SEM) images of PGN−CNT and (b) its electrochemical performance at high rates and low temperatures with DIOX-based and commercial electrolytes. (c) Transmission electron microscopy (TEM) images of GRAL anode and (d) the electrochemical comparison with SLP30 anode at −30 °C and 0.05/0.1 Ag−1.
3.1.2. Other Related Investigations
Recently, energy-dense Li-S batteries were achieved with the assistance of a gel polymer electrolyte, which was designed by redox chemistry between a Li2S cathode and Si anode. The rationally designed CoN@MCNF/Li2S cathode shows good temperature adaptability at around −20 °C [59].
3.2. Electrolyte Chemistry Modulation
Jiao et al. [60] systematically studied the performance of lithium metal anodes over a range of temperatures. A variety of electrolyte systems have been studied, such as a cosolvent of 1,3-dioxolane (DOL)/1,2-dimethoxyethane (DME), and a cosolvent of ethylene carbonate (EC) and dimethyl carbonate (DMC). Both drupe-like and small spherical deposits of lithium were detected at high temperatures of 60 °C. It was shown that between five degrees and minus fifteen degrees, there will be branching of lithium metal anodes. However, at higher temperatures, the C–C and C–O groups of organics in the SEI are enriched, resulting in a low modulus, flexible SEI structure. In thermodynamic and kinetically favored depositional conditions, low-current density, high Coulombic efficiency as well as a nondendritic structure has been achieved in these electrolytes.
Compared with low temperatures, conventional carbonate LiFP6 electrolytes are preferred over poorer SEI layers, with lower Coulombic efficiency and shorter cycle life at high temperature [61]. At 0 °C, a homogenous SEI layer was formed that contained uniformly distributed F-containing species, displaying an extended cycle lifetime and increased Coulombic efficiency (Figure 3). The XRF mappings and micro-XANES images illustrate that F and O are inhomogeneously distributed over most of the surface and from the surface to the bulk, which is important to understand. In particular, when the cycle temperature reaches below 60 °C, the internal composition of the SEI is mainly composed of inorganic Li2CO3, and the surface composition is mainly composed of organic oxygen and LiF. These species are produced by the reaction between the electrolyte and the deposited lithium, thus causing rapid failure when building a thick SEI.
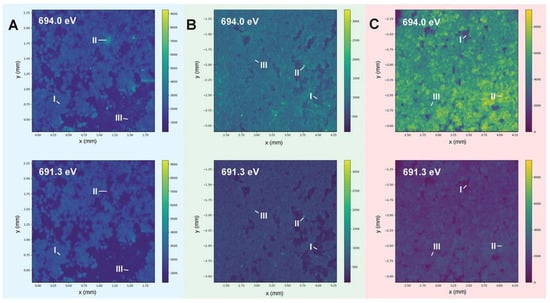
Figure 3. Investigations on temperature effects on the SEI structure using advanced techniques. (A–C) Displays the synchrotron energy-dependent XRF mappings of the cycled lithium metal anode at 0, 25, and 60 °C, respectively.
Robert [62] and co-workers demonstrated that using the positive temperature gradient method (the positive electrode is set to 40 °C and the negative electrode is set to 0 °C) could produce uniform lithium quiescence and reduce deleterious side reactions. In typical LiTFSI-LiNO3-based ether electrolytes, after one hundred cycles, the symmetrical cell with positive thermal gradient exhibited the lowest voltage hysteresis and the longest cycle life. Whereas, under negative temperature gradient conditions (negative pole set to 0 °C and positive pole set to 40 °C), a rapid degradation of battery performance was monitored after only 15 cycles. It demonstrated that the very first lithium seeding state enabled control of quality and cycle life of Li metal anodes. In particular, negative temperature gradients can lead to cold seeding and thermal stripping processes, which generally produce inhomogeneous coatings with high aspect ratios, whereas positive temperature gradients can produce uniform initial lithium quiescence with good long-term cycling performance. These external temperature gradient methods are conducive to stabilizing Li metal anodes.
To achieve an ultra-low temperature lithium metal anode, Xia [63] and co-workers developed a cosolvent electrolyte system by adding electrochemically “inert” dichloromethane (DCM) as diluent to a concentrated ethyl acetate (EA)-based electrolyte. By adding DCM diluent, the solvated structure was changed. In EA solution, the mobile DCM molecules wrap the salt clusters of LiTFSI. Due to this unique structure, the electrolyte exhibits high ionic conductivity, low viscosity, and low-temperature performance. Although the stripping/plating performance of the Li metal anode was not shown, the Li//PI battery exhibited good cycling performance at −70 °C and good performance at low temperatures. However, based on the investigation of the Golmohammad group, the newly fabricated solid-state electrolytes show good stripping/plating performance toward the metallic Li anode under such conditions [64,65,66].
3.3. Artificial SEI Layer
The problem of the high lithium ion desolubilization energy barrier at low temperatures can be effectively mitigated by artificially adjusting the SEI film. The slow process at the lithium anode occurs when the solvated Li attempts to enter the anode structure, where it must peel off its primary solvated sheath [67]. This dynamically challenging process, often referred to as the “charge transfer” component due to its characteristic 50–70 kJ mol−1 activation energy barrier, is the fundamental reason why LIBs cannot be charged at low temperatures. However, the activation energy of the Li ions’ interfacial transfer is different in the presence of different surface films (SEI), and the dynamics of cathode–interface Li ion transfer are also affected by the composition of SEI film [68,69]. Therefore, the artificially modified SEI film effectively affects the “charge transfer” process, providing a beneficial strategy to solve the high Li ion desolubility barrier problem (Figure 4).
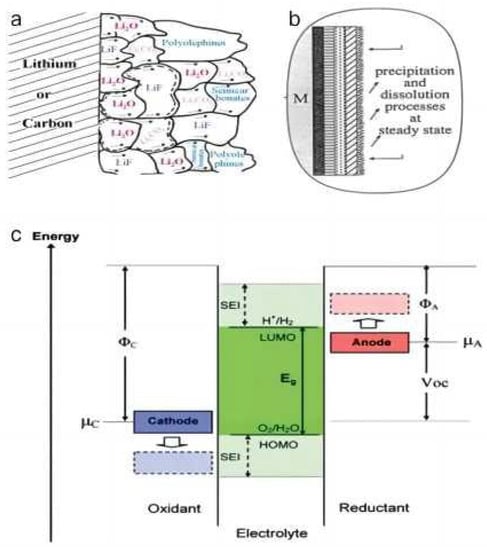
Figure 4. Schematic illustration of the proposed (a) mosaic structure, (b) multilayered structure of the SEI. (c) Schematic open-circuit energy diagram of battery electrolyte. ΦA and ΦC are the anode and cathode work functions. Eg is the window of the electrolyte for thermodynamic stability. A µA > LUMO and/or a µC < HOMO requires a kinetic stability for the formation of an SEI layer.
3.4. The 3D Current Collector Hosts and/or Lithophilic Site Modification
The 3D current collector hosts and lithophile site modification are effective to achieve high-performance lithium anodes and fast conversion kinetics [45]; their feasibility and reliability in extreme hot and cold conditions, however, have been studied relatively infrequently. Peng and co-workers [72] used chemical vapor deposition to produce graphite-coated Ni-Fe foam (graphite @Ni-Fe). It exhibited good cycle performance, and the capacity retention rate was 98.10% after 100 cycles at −50 °C and 0.4 A cm−2, with a Coulomb efficiency over 98.3%. At present, zinc batteries are performing at low temperature, but lithium batteries are not.
This entry is adapted from the peer-reviewed paper 10.3390/ma16124359
This entry is offline, you can click here to edit this entry!
