Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Virus-like particles (VLPs) are formed by self-assembly in insect cells infected with a recombinant baculovirus. Polyarginine/cysteine-tagged antigens are linked to the VLP by a reversible disulfide bond. The VLP possesses self-adjuvanting properties due to the immunostimulatory activity of papillomavirus VLPs. Polyionic VLP vaccines induce robust CD8+ T cell responses in peripheral blood and tumor tissues.
- virus-like particle (VLP)
- papillomavirus
- vaccine
- cross-priming
- cancer vaccine
1. Production of Polyionic Bovine Papillomavirus L1 Virus-Like Particles
The full-length open reading frame (ORF) of bovine papillomavirus (BPV) type 1 L1 (GenBank: BBB04658.1) preceded by a Kozak consensus sequence, with codons modified for efficient expression in insect cells, was constructed by PCR-based gene synthesis. The ORF contained the insertion of a peptide with eight glutamic acid residues and a cysteine residue (E8C) and the deletion of nine wildtype amino acids in the HI loop (aa347-355 were deleted) (Figure 1).
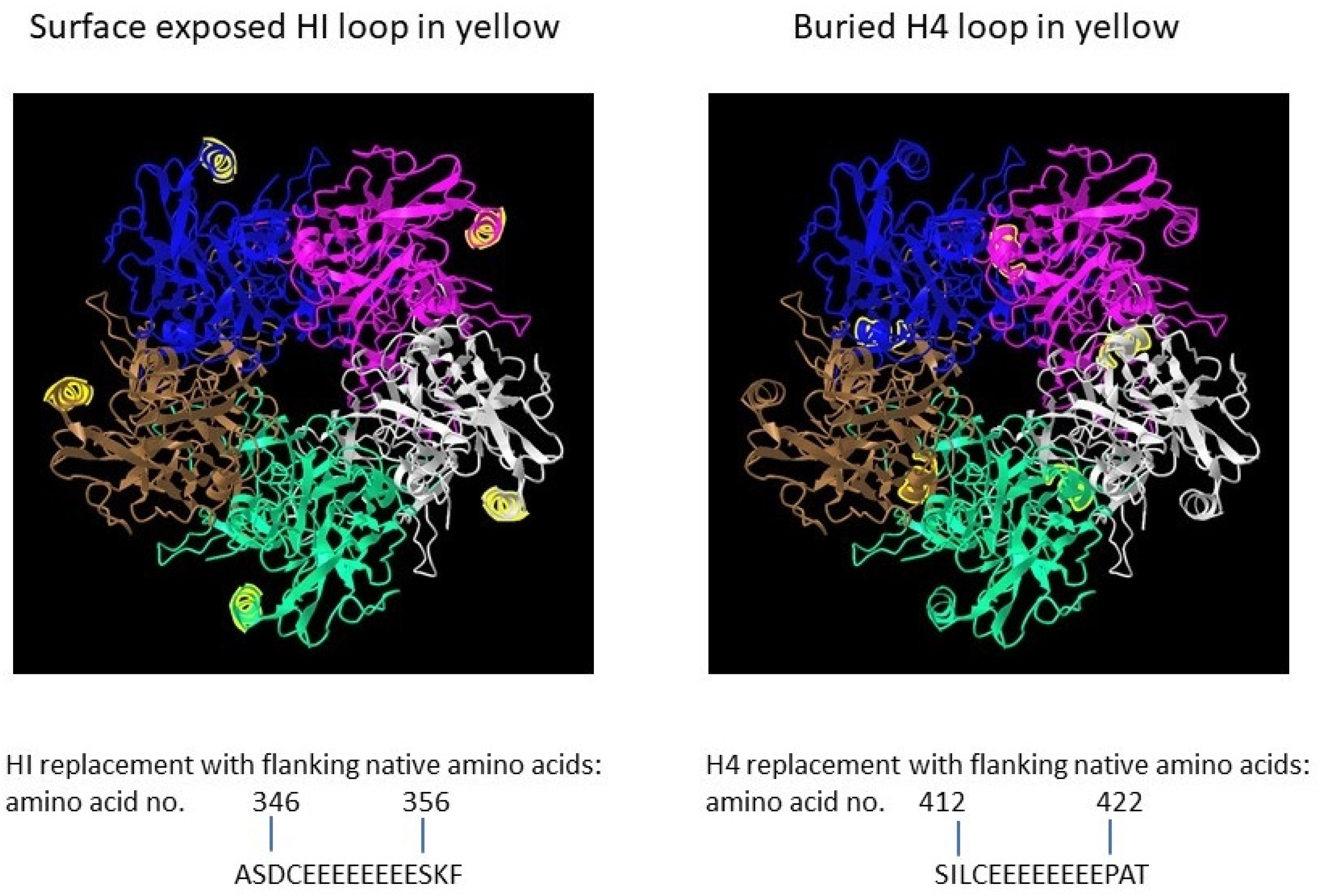
Figure 1. Papillomavirus pentamer highlighting the surface exposed location of the HI loop and the buried location of the H4 loops. The capsomeric structure is that of human papillomavirus type 11 L1 (PDB: 2R5K; PDB DOI: 10.2210/pdb2R5K/pdb [1]), which is closely related to that of bovine papillomavirus L1. Location numbers and flanking amino acid sequences are from capsid protein L1 of delta papillomavirus 4 (accession number: NP_056744.1). The 5 L1 proteins forming the pentamer are colored blue, magenta, white, green and brown. The HI loop region is in yellow.
To evaluate effect of particle size on immunogenicity, an L1 ORF with the deletion of wild-type amino acids and the insertion of E8C in the H4 loop (aa413-421 were deleted) was also constructed [2]. The position of the H4 loop modification disrupts inter-capsomeric interactions necessary for capsid assembly. The modified BPV L1 genes were subcloned into a baculovirus transfer vector, and each transfer vector was co-transfected with linear baculovirus DNA in Spodoptera frugiperda sf9 cells. For the production of virus-like particles (VLPs), Trichoplusia ni (High Five) cells were infected with a high-titer recombinant baculovirus stock. VLPs were purified from infected insect cells by cycles of freezing and thawing in the presence of protease inhibitors. The clarified cell lysate was extracted with an inorganic solvent (Vertrel DF) and layered over 40% sucrose. The sucrose pellet was resuspended in a high salt buffer, incubated with Salt Active Nuclease (Articzyme), and dialyzed into a storage buffer containing 0.5 M NaCl, Tween 80, carboxymethyl cellulose, and FeCl2. Analysis of the purified preparations by electron microscope confirmed that the HI-loop-engineered L1 protein formed a fully assembled VLP with an approximate size of 45–55 nm and the H4-engineered loop formed capsomeres with approximate size of 4–5 nm [2].
2. Formulation of Polyionic Virus-Like Particle Vaccines
The VLP exposes, on the surface, a motif of glutamic acids and a cysteine residue that allows the linkage of antigens of a diverse length, containing a N-terminal tag of eight arginine residues with a single C-terminal cysteine residue or flanking cysteine residues. The linkage reaction involves electrostatic interactions between the charged residues of the tag (polyarginine) and VLP (polyglutamic acid) and the formation of a reversible disulfide bond by an oxidation reduction reaction. Chemically synthesized tagged peptides ranging in size from 9 amino acids to 40 amino acids have been linked to the VLP. The C terminus of the tag consists of two alanine amino acids and a tyrosine or variations thereof as a processing signal for proteases active in MHC class I presentation. Prior to the conjugation reaction, peptide antigens were reduced with Bond-breaker TCEP solution for 20 min at 50 °C. Stock VLP preparations were dialyzed into a physiological salt buffer at 1 mg/mL prior to conjugation. VLPs and peptides at peptide to L1 protein ratios between 4:1 to 8:1 were incubated overnight at 37 °C in the presence of a 5:1 ratio of glutathione disulfide (GSSG) to reduced glutathione (GSH). After the conjugation reaction, VLP vaccine preparations were dialyzed in high salt buffer containing Tween 80, carboxymethyl cellulose, and a 10:1 ratio of GSSG to GSH, aliquoted and stored at −20 °C. The amount of peptide bound to the VLP was determined by ELISA using an antigen-specific monoclonal antibody or by SDS-PAGE analysis. VLPs linked to a MUC1 peptide were analyzed with immunogold-labeled anti-MUC1 monoclonal antibody. Electron microscopy verified the integrity of the VLPs and the successful attachment of the MUC1 peptide [2].
3. Immunological Properties of Polyionic Virus-Like Particles
Polyionic VLPs alone and polyionic VLPs conjugated to a peptide antigen retain the immunological properties of papillomavirus VLPs [2]. BMDC exposed to polyionic VLPs (BPV-HI-E8c unconjugated) or polyionic VLPs linked to a MUC1 peptide antigen (BPV-HI-E8c-MUC1 (conjugated) and polyionic capsomeres (BPV-H4-E8c) or polyionic capsomeres linked to MUC1 (BPV-H4-E8c-MUC) significantly increased the expression of activation and maturation molecules (CD40, CD86, CD80, and MHC class II), with levels only slightly less than that induced by wild-type BPV VLPs. The BPV-H4-E8c capsomeres induced an increase in some activation markers, but the response was lower than that of fully formed VLPs. BMDC exposed to antigen-conjugated or unconjugated polyionic VLPs also produced IL-12p40, but at a level lower than induced by wild-type BPV VLPs. In contrast, polyionic capsomere VLPs very weakly stimulated the production of IL-12p40. To evaluate the ability of polyionic VLPs to activate T cells, splenocytes from MUC1-specific TCR transgenic mice, which provide the APC and a high frequency of naive MUC1-specific T cells, were cultured with a soluble MUC1 peptide or with MUC1-conjugated or unconjugated polyionic VLPs. Following culture with the MUC1 decorated polyionic BPV particles, but not with unconjugated polyionic BPV particles, MUC1-specific TCR transgenic splenocytes secreted significant amounts of IFNγ [2]. Polyionic capsid HI VLPs and polyionic capsomere H4 VLPs induced comparable levels of IFNγ. The responses to MUC1 conjugated to polyionic VLPs were significantly higher than induced by free MUC1 peptide.
4. Comparison of Immunogenicity of Polyionic Virus-Like Particles and Other Vaccine Platforms
4.1. Prostate Tumor Antigens
Comparison of immunogenicity across vaccine platforms is complicated by differences in assays to measure antigen-specific T cell responses and the lack of standardization of these assays. No other vaccine technologies have been formulated with the SPAS1 neoantigen of TRAMP mice, and only one other research group has constructed a vaccine formulated with the prostate stem cell antigen (PSCA) [3]. In that study, the immunization of mice with a DNA vaccine prime and a Venezuelan Equine Encephalitis virus self-amplifying mRNA vaccine boost resulted in a mean frequency of ~240 spot (ELISPOT)-forming cells per 106 splenocytes. This response was much lower than the 2.9% antigen-specific CD8+ T cell response to polyionic VLP vaccination, assuming that 240 spots per million splenocytes corresponded to ~0.3% antigen-specific CD8+ T cells by intracellular cytokine stain (ICS) assay (based on assumption that 25% of splenocytes are CD3+ T cells, and one-third of these cells are CD8+). In a similar study, a comparable prostate tumor antigen, STEAP1, generated an antigen-specific CD8+ T cell response of 1.14% by ICS assay following immunization with a recombinant Chimpanzee adenovirus prime and vaccinia boost [4].
4.2. HPV 16 E7 Antigens
Historically, HPV 16 E7 is one of the most studied tumor antigens. In a comparative study of DNA vaccine constructs formulated with HPV 16 E7, the most potent construct was a plasmid encoding a calreticulin (CRT)-E7 fusion protein. Immunization of mice with this construct generated ~924 antigen-specific IFNγ-secreting CD8+ T cells per 3 × 105 splenocytes, which corresponded to an approximately 3.7% frequency of antigen-specific CD8+ T cells [5]. In a BioNTech SE study of an mRNA vaccine encoding the HPV 16 E7 protein, a 4% frequency of E7-specific splenic CD8+ T cells was reported [6]. E7 peptide antigens have also been delivered with nanoparticles. A hydrophilic polyester nanoparticle loaded with E7 peptide and adjuvanted with poly I:C induced a 1% frequency of E7 specific CD8+ T cells [7]. A 30nm polymeric nanoparticle with a surface exposed cysteine residue was formulated with a thiol containing E7 peptide and administered with a CpG adjuvant. The vaccine induced a 9% frequency of E7 specific splenic T cells [8]. Of note, this nanoparticle used a reversible disulfide bond for attachment of antigen to the VLP. However, unlike polyionic VLPs, an exogenous adjuvant (CpG) had to be used in order to induce an immune response. CpG poses a potential risk for adverse events in human studies.
It has been researcher's experience that the CD8+ T cell response to immunization with polyionic HPV 16 E7 peptide VLPs can be ~8%, and, after a second immunization 3 months following the first, the response can be up to 22% antigen-specific CD8+ T cells (Figure 2). Collectively, these studies demonstrated that the immunogenicity of polyionic VLPs was superior to, or as good as, other vaccine platforms formulated with the same antigen.
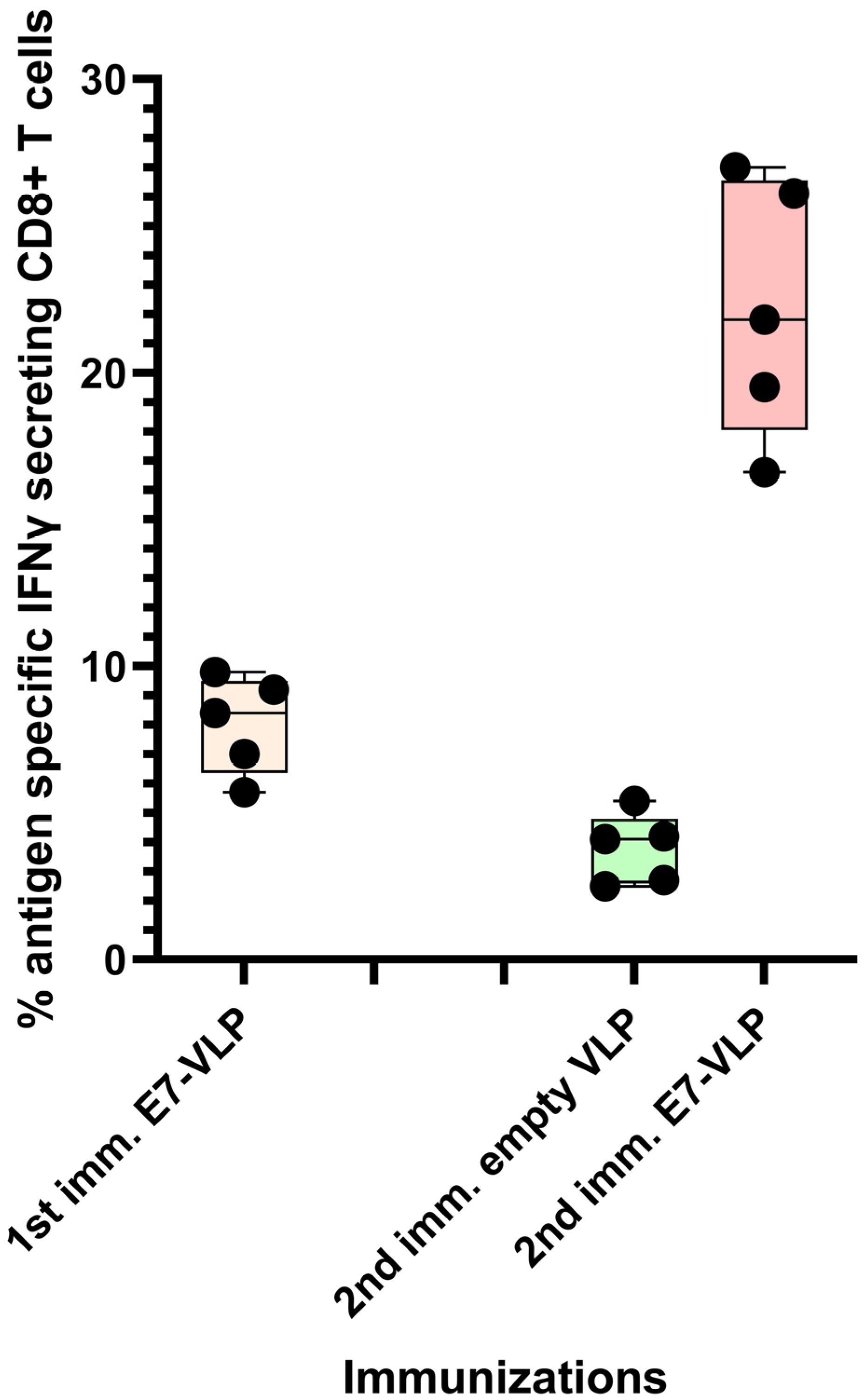
Figure 2. Primary immunization with polyionic E7 VLP vaccine and repeated immunization after 3 months. In total, 15 C57BL6 mice were immunized weekly three times × by intradermal injection with a polyionic VLP vaccine formulated with HPV type 16 H-2Db-restricted E7 epitope (E749-57; RAHYNIVTF). In total, 5 mice were euthanized 10 days after the last dose of vaccine, and the frequency of splenic antigen-specific CD8+ T cell was measured by ICS assay. After 3 months, 5 mice were reimmunized with the polyionic E7 VLP vaccine, and another 5 mice were immunized with an empty polyionic VLP as a control. Mice were euthanized 10 days after the last dose of vaccine to measure splenic antigen-specific CD8+ T cells. The response to reimmunization was significantly higher than that after to primary immunization (Mann–Whitney test, p = 0.008), whereas the response after re-immunization with empty VLPs was significantly lower, consistent with contraction of the CD8+ T cell response over time.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24129851
References
- Bishop, B.; Dasgupta, J.; Klein, M.; Garcea, R.L.; Christensen, N.D.; Zhao, R.; Chen, X.S. Crystal structures of four types of human papillomavirus L1 capsid proteins: Understanding the specificity of neutralizing monoclonal antibodies. J. Biol. Chem. 2007, 282, 31803–31811.
- Pejawar-Gaddy, S.; Rajawat, Y.; Hilioti, Z.; Xue, J.; Gaddy, D.F.; Finn, O.J.; Viscidi, R.P.; Bossis, I. Generation of a tumor vaccine candidate based on conjugation of a MUC1 peptide to polyionic papillomavirus virus-like particles. Cancer Immunol. Immunother. 2010, 59, 1685–1696.
- Garcia-Hernandez, M.L.; Gray, A.; Hubby, B.; Klinger, O.J.; Kast, W.M. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res. 2008, 68, 861–869.
- Cappuccini, F.; Stribbling, S.; Pollock, E.; Hill, A.V.S.; Redchenko, I. Immunogenicity and efficacy of the novel cancer vaccine based on simian adenovirus and MVA vectors alone and in combination with PD-1 mAb in a mouse model of prostate cancer. Cancer Immunol. Immunother. 2016, 65, 701–713.
- Kim, J.W.; Hung, C.-F.; Juang, J.; He, L.; Kim, T.W.; Armstrong, D.K.; Pai, S.I.; Chen, P.-J.; Lin, C.-T.; Boyd, D.A.; et al. Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene Ther. 2004, 11, 1011–1018.
- da Silva, J.R.; Bitencourt Rodrigues, K.; Formoso Pelegrin, G.; Silva Sales, N.; Muramatsu, H.; de Oliveira Silva, M.; Porchia, B.F.M.M.; Moreno, A.C.R.; Aps, L.R.M.M.; Venceslau-Carvalho, A.A.; et al. Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci. Transl. Med. 2023, 15, eabn3464.
- Rahimian, S.; Fransen, M.F.; Kleinovink, J.W.; Christensen, J.R.; Amidi, M.; Hennink, W.E.; Ossendorp, F. Polymeric nanoparticles for co-delivery of synthetic long peptide antigen and poly IC as therapeutic cancer vaccine formulation. J. Control. Release 2015, 203, 16–22.
- Galliverti, G.; Tichet, M.; Domingos-Pereira, S.; Hauert, S.; Nardelli-Haefliger, D.; Swartz, M.A.; Hanahan, D.; Wullschleger, S. Nanoparticle Conjugation of Human Papillomavirus 16 E7-long Peptides Enhances Therapeutic Vaccine Efficacy against Solid Tumors in Mice. Cancer Immunol. Res. 2018, 6, 1301–1313.
This entry is offline, you can click here to edit this entry!
