Transarterial chemoembolization (TACE) has been standard treatment for intermediate-stage hepatocellular carcinoma (HCC). However, all intermediate-stage HCC patients did not benefit from TACE treatment because intermediate-stage HCC encompasses a wide variety of HCCs. Owing to remarkable progress in systemic therapy, including molecular-targeted therapy for advanced-stage HCC, the standard treatment of HCC has recently shifted to systemic therapy.
- hepatocellular carcinoma
- intermediate-stage hepatocellular carcinoma
- molecular-targeted therapy
- tyrosine kinase inhibitor
- immune checkpoint inhibitor
- atezolizumab plus bevacizumab
- durvalumab plus tremelimumab
1. Introduction
2. The Definition and Standard Treatment of Intermediate-Stage HCC
2.1. Effectiveness of TACE in Intermediate-Stage HCC Treatment
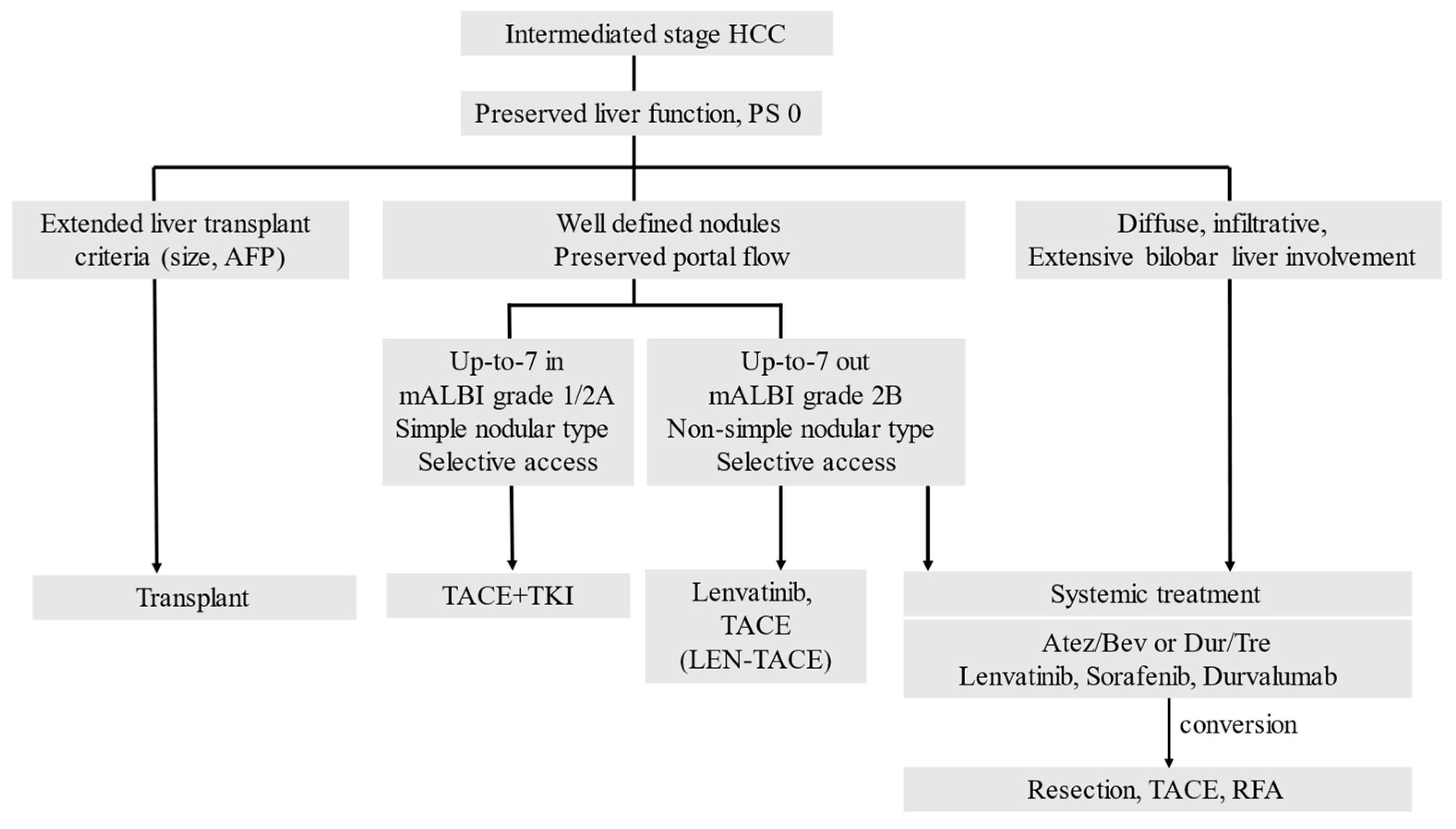
2.2. Subclassification of Intermediate-Stage HCC
2.3. Switching from TACE to Drug Therapy as Treatment for Intermediate-Stage HCC
3. Advances in Cancer Immunotherapy
4. Combination of TACE and Molecular-Targeted Drugs
Systemic therapy using a highly responsive agent such as lenvatinib (LEN) in combination with subsequent selective TACE for residual tumors enhances the curative effect of TACE, preserves liver function, suppresses hypoxia-induced cytokines, and ultimately improves the patients’ survival. Therefore, the Asian Pacific Experts on Primary Liver Cancer (APPLE) [23] and the Japanese Society of Hepatology (JSH) [24] consensus statements recommend LEN as the first-line treatment for patients with intermediate-stage HCC who are not suitable for TACE. Recently, clinical practice guidelines from the E-updated European Society for Medical Oncology recommended upfront systemic therapy for patients who are ineligible for TACE [25].
The combined effect of TACE and molecular-targeted drugs can be explained as follows: the molecular-targeted drug acts on residual tumors caused by TACE, exerting anti-angiogenic and anti-tumor growth effects to prevent the exacerbation of residual tumors, avoid the development of new intrahepatic lesions, and suppress vascular invasion and distant metastasis. Molecular-targeted drugs with an anti-VEGF effect act on tumor blood vessels to improve the vascular permeability; reduce the tumor interstitial pressure; improve the drug delivery; enhance the treatment efficacy [24]; and suppress the effect of the transient increase of VEGF immediately after the occurrence of TACE-related ischemia, which is considered as one of the causes of tumor exacerbation [23][24][26][27]. Although multiple clinical trials investigating the efficacy of combination therapy with TACE and molecular-targeted drugs have been conducted, their efficacy has not been demonstrated. Under these circumstances, the TACTICS trial (TACE therapy in combination with sorafenib), which investigated the combined effect of sorafenib and lipiodol TACE, showed the potential effects of treatment with a combination of targeted drugs and TACE.
Sorafenib-TACE sequential therapy also extended the OS by 3.7 months compared with TACE alone (35.6 months vs. 31.9 months, HR: 0.924). These data indicate that sequential therapy with sorafenib and TACE was more effective than TACE alone in prolonging the PFS [28]. Although the TACTICS treatment improved the PFS, it did not significantly extend the OS. For this reason, 76.3% of the patients who received TACE alone also received post-trial treatment, and nearly half of these patients received sorafenib. Therefore, patients generally received aggressive post-trial treatment, which can extend the post-progression survival (PPS) and weaken the benefit obtained from the original trial treatment [29].
5. New Treatment Strategy for Intermediate Stage HCC: TACE plus Cancer Immunotherapy, and Conversion Therapy
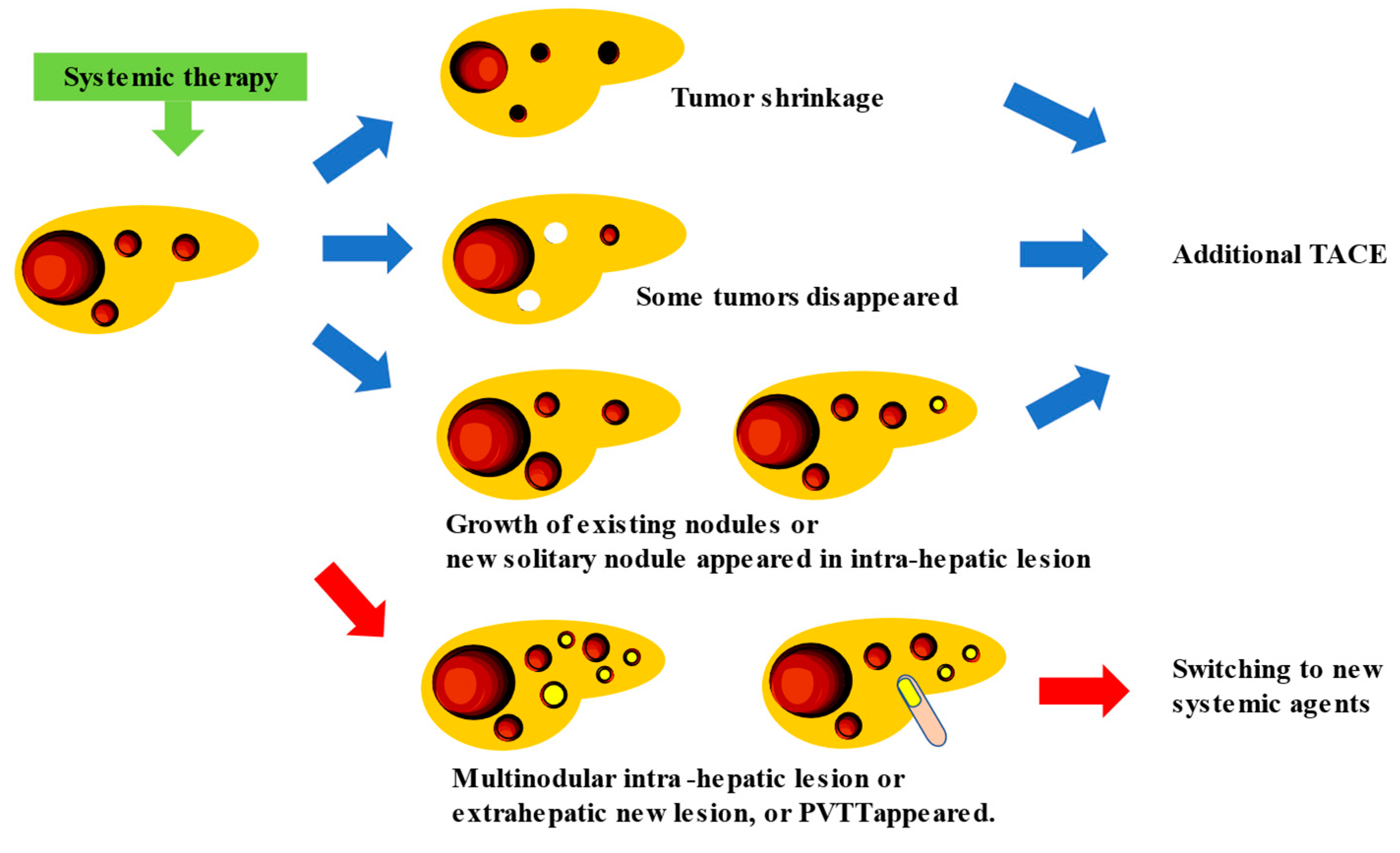
This entry is adapted from the peer-reviewed paper 10.3390/cancers15061798
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33.
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390.
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo controlled trial. Lancet Oncol. 2009, 10, 25–34.
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66.
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomized phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173.
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296.
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63.
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905.
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Dao, T.V. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070.
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750.
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6.
- Lo, C.M.; Ngan, H.; Tso, W.K.; Liu, C.L.; Lam, C.M.; Poon, R.T.; Fan, S.T.; Wong, J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002, 35, 1164–1171.
- Llovet, J.M.; Real, M.I.; Montaña, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Solà, R.; et al. Arterialembolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 2002, 359, 1734–1739.
- Bolondi, L.; Burroughs, A.; Dufour, J.F.; Galle, P.R.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Sangro, B. Heterogeneityof patients with intermediate (BCLC B) Hepatocellular Carcinoma: Proposal for a subclassification to facilitate treatment decisions. Sem Liver Dis. 2012, 32, 348–359.
- Yamakado, K.; Miyayama, S.; Hirota, S.; Mizunuma, K.; Nakamura, K.; Inaba, Y.; Yamamoto, S.; Matsuo, K.; Nishida, N.; Aramaki, T.; et al. Prognosis of patients with intermediate-stage hepatocellular carcinomas based on the Child-Pugh score: Subclassifying the intermediate stage (BarcelonaClinic Liver Cancer stage B). Jpn. J. Radiol. 2014, 32, 644–649.
- Kudo, M.; Arizumi, T.; Ueshima, K.; Sakurai, T.; Kitano, M.; Nishida, N. Subclassificationof BCLC B Stage Hepatocellular Carcinoma and Treatment Strategies: Proposal of Modified Bolondi’s Subclassification (Kinki Criteria). Dig. Dis. 2015, 33, 751–758.
- Arizumi, T.; Ueshima, K.; Iwanishi, M.; Minami, T.; Chishina, H.; Kono, M.; Takita, M.; Kitai, S.; Inoue, T.; Yada, N.; et al. Validationof Kinki Criteria, a Modified Substaging System, in Patients with Intermediate Stage Hepatocellular Carcinoma. Dig. Dis. 2016, 34, 671–678.
- Arizumi, T.; Ueshima, K.; Minami, T.; Kono, M.; Chishina, H.; Takita, M.; Kitai, S.; Inoue, T.; Yada, N.; Hagiwara, S.; et al. Effectiveness of Sorafenib in Patients with Transcatheter Arterial Chemoembolization (TACE) Refractory and Intermediate-Stage Hepatocellular Carcinoma. Liver Cancer 2015, 4, 253–262.
- Kelley, R.K.; Rimassa, L.; Cheng, A.L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008.
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A hemoembol, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90.
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III Trial. J. Clin. Oncol. 2020, 38, 193–202.
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693.
- Kudo, M.; Han, K.H.; Ye, S.L.; Zhou, J.; Huang, Y.H.; Lin, S.M.; Wang, C.K.; Ikeda, M.; Chan, S.L.; Choo, S.P.; et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific primary liver cancer expert consensus statements. Liver Cancer. 2020, 9, 245–260.
- Kudo, M.; Kawamura, Y.; Hasegawa, K.; Tateishi, R.; Kariyama, K.; Shiina, S.; Toyoda, H.; Imai, Y.; Hiraoka, A.; Ikeda, M.; et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021, 10, 181–223.
- Vogel, A.; Martinelli, E.; ESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO clinical practice guidelines. Ann. Oncol. 2021, 32, 801–805.
- Kawamura, Y.; Kobayashi, M.; Shindoh, J.; Kobayashi, Y.; Okubo, S.; Tominaga, L.; Kajiwara, A.; Kasuya, K.; Iritani, S.; Fujiyama, S.; et al. Lenvatinib-Transarterial Chemoembolization Sequential Therapy as an Effective Treatment at Progression during Lenvatinib Therapy for Advanced Hepatocellular Carcinoma. Liver Cancer 2020, 9, 756–770.
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62.
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Final Results of TACTICS: A Randomized, Prospective Trial Comparing Transarterial Chemoembolization Plus Sorafenib to Transarterial Chemoembolization Alone in Patients with Unresectable Hepatocellular Carcinoma. Liver Cancer 2022, 11, 354–367.
- Kudo, M. Implications of the TACTICS Trial: Establishing the New Concept of Combination/Sequential Systemic Therapy and Transarterial Chemoembolization to Achieve Synergistic Effects. Liver Cancer 2022, 11, 487–496.
- Aoki, T.; Kudo, M.; Ueshima, K.; Morita, M.; Chishina, H.; Takita, M.; Hagiwara, S.; Ida, H.; Minami, Y.; Tsurusaki, M.; et al. Exploratory Analysis of Lenvatinib Therapy in Patients with Unresectable Hepatocellular Carcinoma Who Have Failed Prior PD-1/PD-L1 Checkpoint Blockade. Cancers 2020, 12, 3048.
- Hiraoka, A.; Kumada, T.; Tada, T.; Hirooka, M.; Kariyama, K.; Tani, J.; Atsukawa, M.; Takaguchi, K.; Itobayashi, E.; Fukunishi, S.; et al. Early experience of atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma BCLC-B stage patients classified as beyond up to seven criteria-Multicenter analysis. Hepatol. Res. 2022, 52, 308–316.
- Kudo, M.; Finn, R.S.; Galle, P.R.; Zhu, A.X.; Ducreux, M.; Cheng, A.; Likeda, M.; Tsuchiya, K.; Aoki, K.; Jia, J.; et al. Imbrave150: Efficacy and Safety of Atezolizumab plus Bevacizumab versus Sorafenib in Patients with Barcelona Clinic Liver Cancer Stage B Unresectable Hepatocellular Carcinoma—An Exploratory Analysis of the Phase III Study. Liver Cancer 2022.
- Kudo, M. A Novel Treatment Strategy for Patients with Intermediate-Stage HCC Who Are Not Suitable for TACE: Upfront Systemic Therapy Followed by Curative Conversion. Liver Cancer 2021, 10, 539–544.
- Facciorusso, A.; Bellanti, F.; Villani, R.; Salvatore, V.; Muscatiello, N.; Piscaglia, F.; Vendemiale, G.; Serviddio, G. Transarterial chemoembolization vs. bland embolization in hepatocellular carcinoma: A meta-analysis of randomized trials. United Eur. Gastroenterol. J. 2017, 5, 511–518.
- Reig, M.; Rimola, J.; Torres, F.; Darnell, A.; Rodriguez-Lope, C.; Forner, A.; Llarch, N.; Ríos, J.; Ayuso, C.; Bruix, J. Postprogression survival of patients with advanced hepatocellular carcinoma: Rationale for second-line trial design. Hepatology 2013, 58, 2023–2031.
- Billan, S.; Kaidar-Person, O.; Gil, Z. Treatment after progression in the era of immunotherapy. Lancet Oncol. 2020, 21, e463–e476.
