Atrial fibrillation (AF) is the most common cause of hospital admission among all arrhythmias in the general population. Moreover, AF represents the most common arrhythmia in the athletic population as well. The complex but fascinating relationship between sport and atrial fibrillation has not yet been fully clarified. Although the benefits of moderate physical activity in controlling cardiovascular risk factors and in reducing the risk of atrial fibrillation have been widely demonstrated, some concerns have been raised about the potential adverse effects of physical activity. Endurance activity in middle-aged men athletes appears to increase the risk of AF. Several different physiopathological mechanisms may explain the increased risk of AF in endurance athletes, including the imbalance of the autonomic nervous system, changes in left atrial size and function and presence of atrial fibrosis.
1. Introduction
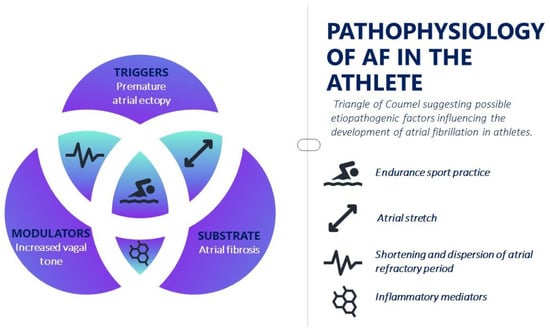
Although the physiopathology scenario of AF is complex, the initiation and maintenance of this arrhythmia is commonly due to a focus that determines rapid supraventricular extrasystoles or through re-entry circuits triggered by ectopic foci, either as a result of late post-depolarization or after early post-depolarization. Today, Coumel’s triangle still represents the clearest method of understanding the complex pathophysiological AF mechanism, identifying three important elements: electrical and structural remodeling, triggers and the role of the autonomic nervous system (Figure 1). Structural and electrical changes within the atrium that lead to a reduction in atrial refractoriness and therefore to conduction slowdown and electrical dispersion are known to facilitate the formation of re-entry circuits and atrial fibrillation as a result.
Figure 1. Pathophysiology of atrial fibrillation in the athlete.
2. Autonomic Nervous System Changes
AF persistence is further facilitated by changes in the autonomic nervous system that are able to exacerbate an ectopic focus (sympathetic and parasympathetic activation) or shorten the atrial refractory period (parasympathetic activation). Although few studies have evaluated atrial ectopic beat activity in athletes, there is some scientific evidence that the extra-systolic burden may be higher in athletes than in controls. In runners with a longer training history, there was a small but significant increase in premature atrial contractions compared with those performing fewer hours of training
[1]. Similarly, the frequency of premature atrial contractions was higher in professional athletes than in amateur athletes or in a group of sedentary controls matched for age and sex
[2]. However, in both studies, the overall premature atrial contraction burden was low compared to that observed in AF patients, possibly indicating that ectopic atrial activity, at least at rest, may not always play a significant role in inducing AF in athletes. Moreover, in both animal and human models, continuous physical training determines an imbalance of the autonomic nervous system (ANS), resulting in a greater parasympathetic activation and a reduction in the sympathetic tone
[3]. Although such changes are thought to be cardioprotective, they may increase atrial susceptibility to arrhythmia, mainly as a result of a shorter atrial refractory period dependent on potassium currents mediated by acetylcholine release. It has also been shown that abrupt changes in the sympathovagal balance, often observed at the start of exercise and during recovery, may precede AF onset. Although both parasympathetic and sympathetic tone shorten atrial refractory time, more heterogeneous atrial parasympathetic innervation produces greater arrhythmic susceptibility
[4]. Endurance exercise results in increased vagal activity which could facilitate AF inducibility. In an experimental model in rats undergoing the endurance exercise equivalent to 10 years of training, vagal activity was increased as expected, predisposing them to AF, but rapidly decreased after a period of detraining
[3]. In contrast, another subsequent study in mice undergoing physical exercise showed that the inhibition of inflammatory mediators, such as TNF-alpha, reduced exercise-induced negative remodeling, resulting in a decreased AF inducibility, but had no effect on positive remodeling, including increased vagal tone. These data suggest that factors other than the ANS also contribute to the pathogenesis of sports-induced AF
[5].
3. Inflammation and Oxidative Stress
Inflammation plays an important role in promoting and maintaining atrial fibrillation, involving both structural and electrophysiological alterations that lead to atrial remodeling. In endurance athletes, it is well known that strenuous and continuous exercise may promote a generalized pro-inflammatory status, depending on the quantity and quality of exercise not followed by appropriate rest. The inflammatory state is mediated by cytokines such as IL-6, IL-8, and TNF-α; after a mechanical or biochemical myocardial insult, those pro-inflammatory cytokines promote the recruitment of immune cells that infiltrate the atrial myocardium. Monocytes, Th2, and mastocytes, in the late stage of inflammation, promote tissue fibrosis by secreting profibrotic factors such as TGFβ and enhance the activation and differentiation of fibroblasts in myofibroblasts
[6]. In addition, profibrotic factors induce augmented ROS (reactive oxygen species) production through mitochondria, ending in the activation of p38 and ERK1/2 pathways, enhancing the transcription of fibrotic genes. ROS are normally produced by mitochondria through oxygen metabolism, but an excessive increase may directly affect ion channels and the propagation of action potential
[7] .
4. Atrial Enlargement and Fibrosis
Structural remodeling, in terms of left atrial enlargement, seems to predispose an individual to AF and this occurs in endurance sports as an adaptation to exercise. The increase in atrial size is probably determined by the hemodynamic stress of high-intensity exercise, where maximum cardiac output often reaches 30 L/min. During exercise, both right atrial pressure and pulmonary capillary pressure are markedly high, and it is likely that over time, with prolonged exercise, this leads to ventricular enlargement. A study based on echocardiographic two-dimensional speckle tracking (STE) aimed to characterize myocardial deformation in master athletes with a history of paroxysmal AF, has shown that elite athletes have significant differences in the left atrial function when compared with controls. In fact, a normal left atrial reservoir function but a reduced left atrial contribution to left ventricular diastolic filling was observed. This phenomenon seems to be associated with normal and even super-normal diastolic function and is accompanied by the shift of the left ventricular filling period towards early diastole, mainly due to an increase in the flexibility, elasticity and distensibility of the left ventricular myocardium. In addition, veteran male athletes had significantly higher LA volumes than non-athletes. In contrast, STE-LA values were similar in athletes and non-athletes with paroxysmal AF and significantly lower than in those without AF, suggesting that LA functional parameters are more closely related to AF than volumetric parameters in veteran athletes
[8]. In addition to atrial enlargement, the presence of fibrosis within the atria is part of the arrhythmic substrate for the development of AF. In humans, the presence of fibrosis or areas of low voltage are significant predictors of recurrent AF in patients undergoing transcatheter ablation. The development of fibrosis after endurance exercise was demonstrated in a mouse training model, where 16 weeks of training resulted in a significant increase in the right and left atrial fibrosis. The development of fibrosis in conjunction with vagal hypertonus led to the increased susceptibility to atrial fibrillation. Curiously, it is not the complete reduction in atrial fibrosis but the reduced inducibility of AF that determines a detraining period, suggesting that atrial fibrosis is a key factor but not the only mechanism contributing to the risk of developing AF
[5].
5. BMI and Diet
Lifestyle factors, including diet, weight and BMI, play a role in AF pathogenesis. Weight and BMI are strictly connected with AF onset, its persistence and even its recurrence after ablation
[9]. BMI and progression from paroxysmal to permanent AF have a direct dose–response relationship in the study by Tsang et al.
[10]. Obesity seems to be an independent risk factor regarding the pro-inflammatory status associated with the condition, and robust data support this hypothesis. Jamaly et al.
[11] provide data regarding the impact of dramatic surgical weight loss on incident AF, and they found that every 5-unit BMI increment raised the risk of incident AF by 33%. On the other hand, in the same study weight loss was independently associated with a reduction in AF risk of 31%. The LEGACY trial
[12] supports the importance of weight loss in AF patients as well: after a rigorous weight loss program, there were higher rates of arrhythmia-free survival. Weight loss appears to be crucial to the point that the scientific community is starting to consider the possible role of surgical weight loss in AF populations. In this light, to be more conservative, diet interventions could be assumed to be of relevance; however, despite much conjecture, little evidence exists to advocate for a specific dietary approach
[13]. The Mediterranean diet supplemented with extra virgin olive oil resulted in a lower incidence of AF in PREDIMED study
[14], but more data are needed to confirm Mediterranean diet superiority. Independent of the dietary approach chosen, a high dietary sodium intake is associated with the long-term risk of new-onset atrial fibrillation and should be minimized
[15]. Alcohol is a well-known risk factor for AF and is estimated that 5% to 10% of all AF cases are caused by alcohol consumption
[16]; emblematic of this is the “Holiday Heart Syndrome”, in which AF onset in structurally normal heart is associated with alcohol abuse. Direct cardiotoxic effects, including inflammation in myocardial cells, are produced by alcohol abuse and upregulated sympathetic tone, which can be one of arrhythmia’s triggers.
6. Exogen Stimulant Agents and Supplements
Stimulant agents, such as guarana, taurine, tea and energy drinks in general, may play a role in AF pathogenesis in terms of their effects on ANS, and some cases of AF have been described in the context of the overconsumption of energy drinks. Athletes might consume a large quantity of energy drinks and/or consume various types of supplements during their sports activity or in the peri-work out period. The American Food and Drug Administration and the European EMA do not fully regulate energy drinks/supplements; there might be certain unreported ingredients, which, in addition to caffeine or alcohol, could enhance the risk of arrhythmias. Despite its stimulant effects, the role of caffeine alone is different. Several trials, including cohort, case–control and observational studies and meta-analyses, have shown no significant association between habitual caffeine consumption and an increased risk of AF. Habitual caffeine drinkers instead display cardioprotective effects due to the blocking of transforming growth factor signaling and LA refractory period lengthening, decreasing the likelihood of arrhythmia development
[17]. Among athletes’ supplements, creatine deserves a special mention, a substance utilized by athletes to enhance exercise performance and help muscular growth. In a case report
[18] and other previous anecdotal reports linking creatine to the development of arrhythmia, creatine monohydrate was found to be a probable cause of the atrial fibrillation in an otherwise healthy 30-year-old Caucasian man. Athletes and in general people at risk for AF should be cautious of supplement consumption as resulting effects are still uncertain.
KEY POINTS
-
Imbalance and changes in ANS may promote AF onset and persistence.
-
Strenuous and continuous exercise seems to cause a generalized pro-inflammatory status, which may promote and maintain AF.
-
Atrial enlargement and fibrosis are part of the arrhythmic substrate for the development of AF.
-
Diet, BMI and body weight act in AF pathogenesis and prognosis.
-
Supplements should be consumed carefully in AF and athlete populations.
This entry is adapted from the peer-reviewed paper 10.3390/jcdd10060255