More than 7000 synthetic compounds known as per- and poly-fluoroalkyl substances (PFAS) are applied to food packaging and other materials to provide fat, fire, and/or water resistance properties. These compounds have exceptional environmental stability and persistence due to the strong C-F chemical bond, earning them the moniker “forever chemicals”. Emission of PFAS from industrial waste leads to water, air, and soil contamination. Due to this ubiquitous nature, combined with the fact that PFAS in humans are known to have carcinogenic and reprotoxic effects and to cause vaccine resistance and depression of the immunity system, PFAS may constitute a major threat to human health. For this reason, the attention of the scientific community and of control bodies is increasing and as a consequence legislation and the scientific literature on PFAS are constantly evolving.
- PFAS
- Toxic effects
- Environment
- Food safety
1. Introduction
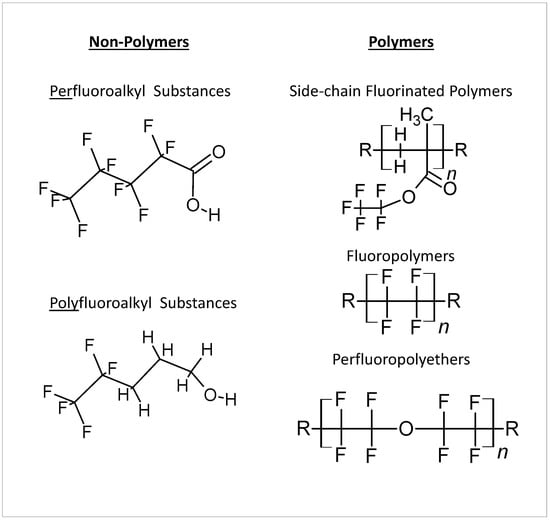
2. Toxic Effects of PFAS in Organisms
This entry is adapted from the peer-reviewed paper 10.3390/app13116696
References
- Available online: https://echa.europa.eu/it/hot-topics/perfluoroalkyl-chemicals-pfas (accessed on 12 May 2023).
- Available online: https://www.niehs.nih.gov/health/topics/agents/pfc/index.cfm (accessed on 12 May 2023).
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; De Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P.J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541.
- Daly, E.R.; Chan, B.P.; Talbot, E.A.; Nassif, J.; Bean, C.; Cavallo, S.J.; Metcalf, E.; Simone, K.; Woolf, A.D. Per- and polyfluoroalkyl substance (PFAS) exposure assessment in a community exposed to contaminated drinking water, New Hampshire, 2015. Int. J. Hyg. Environ. Health 2018, 221, 569–577.
- Jurikova, M.; Dvorakova, D.; Pulkrabova, J. The occurrence of perfluoroalkyl substances (PFAS) in drinking water in the Czech Republic: A pilot study. Environ. Sci. Pollut. Res. 2022, 29, 60341–60353.
- Gazzotti, T.; Sirri, F.; Ghelli, E.; Zironi, E.; Zampiga, M.; Pagliuca, G. Perfluoroalkyl contaminants in eggs from backyard chickens reared in Italy. Food Chem. 2021, 362, 130178.
- Available online: https://trends.google.com/trends/explore?date=all&q=PFAS (accessed on 12 May 2023).
- Available online: https://www.webofscience.com/wos/woscc/basic-search (accessed on 12 May 2023).
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630.
- Vélez, M.P.; Arbuckle, T.E.; Fraser, W.D. Maternal exposure to perfluorinated chemicals and reduced fecundity: The MIREC study. Hum. Reprod. 2015, 30, 701–709.
- Fei, C.; McLaughlin, J.K.; Lipworth, L.; Olsen, J. Maternal levels of perfluorinated chemicals and subfecundity. Hum. Reprod. 2009, 24, 1200–1205.
- Whitworth, K.W.; Haug, L.S.; Baird, D.D.; Becher, G.; Hoppin, J.A.; Skjaerven, R.; Thomsen, C.; Eggesbo, M.; Travlos, G.; Wilson, R.; et al. Perfluorinated compounds and subfecundity in pregnant women. Epidemiology 2012, 23, 257–263.
- Vestergaard, S.; Nielsen, F.; Andersson, A.M.; Hjøllund, N.H.; Grandjean, P.; Andersen, H.R.; Jensen, T.K. Association between perfluorinated compounds and time to pregnancy in a prospective cohort of Danish couples attempting to conceive. Hum. Reprod. 2012, 27, 873–880.
- Buck Louis, G.M.; Sundaram, R.; Schisterman, E.F.; Sweeney, A.M.; Lynch, C.D.; Gore-Langton, R.E.; Maisog, J.; Kim, S.; Chen, Z.; Barr, D.B. Persistent environmental pollutants and couple fecundity: The LIFE study. Environ. Health Perspect. 2013, 121, 231–236.
- Grandjean, P.; Heilmann, C.; Weihe, P.; Nielsen, F.; Mogensen, U.B.; Timmermann, A.; Budtz-Jørgensen, E. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J. Immunotoxicol. 2017, 14, 188–195.
- Abraham, K.; Monien, B.H. Transdermal absorption of 13C4-perfluorooctanoic acid (13C4-PFOA) from a sunscreen in a male volunteer—What could be the contribution of cosmetics to the internal exposure of perfluoroalkyl substances (PFAS)? Environ. Int. 2022, 169, 107549.
- Blomberg, A.J.; Haug, L.S.; Lindh, C.; Sabaredzovic, A.; Pineda, D.; Jakobsson, K.; Nielsen, C. Changes in perfluoroalkyl substances (PFAS) concentrations in human milk over the course of lactation: A study in Ronneby mother-child cohort. Environ. Res. 2023, 219, 115096.
- Available online: https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas#:~:text=EPA%20is%20developing%20a%20proposed,by%20the%20end%20of%202023. (accessed on 12 May 2023).
- Available online: https://www.fda.gov/food/environmental-contaminants-food/and-polyfluoroalkyl-substances-pfas (accessed on 12 May 2023).
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; et al. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020, 18, e06223.
- Jahnke, A.; Marchand, P. Guidance Document on Analytical Parameters for the Determination of Per- and Polyfluoroalkyl Substances (PFAS) in Food and Feed; EURL POPs: Freiburg, Germany, 2022; pp. 1–26.
- Briels, N.; Ciesielski, T.M.; Herzke, D.; Jaspers, V.L.B. Developmental Toxicity of Perfluorooctanesulfonate (PFOS) and Its Chlorinated Polyfluoroalkyl Ether Sulfonate Alternative F-53B in the Domestic Chicken. Environ. Sci. Technol. 2018, 52, 12859–12867.
- Bursian, S.J.; Link, J.E.; McCarty, M.; Simcik, M.F. The Subacute Toxicity of Perfluorooctane Sulfonate and/or Perfluorooctanoic Acid and Legacy Aqueous Film-Forming Foams to Japanese Quail (Coturnix japonica) Chicks. Environ. Toxicol. Chem. 2021, 40, 695–710.
- Bartlett, A.J.; De Silva, A.O.; Schissler, D.M.; Hedges, A.M.; Brown, L.R.; Shires, K.; Miller, J.; Sullivan, C.; Spencer, C.; Parrott, J.L. Lethal and sublethal toxicity of perfluorooctanoic acid (PFOA) in chronic tests with Hyalella azteca (amphipod) and early-life stage tests with Pimephales promelas (Fathead minnow). Ecotoxicol. Environ. Saf. 2021, 207, 111250.
- Zhang, L.; Niu, J.; Li, Y.; Wang, Y.; Sun, D. Evaluating the sub-lethal toxicity of PFOS and PFOA using rotifer Brachionus calyciflorus. Environ. Pollut. 2013, 180, 34–40.
- Shi, X.; Du, Y.; Lam, P.K.S.; Wu, R.S.S.; Zhou, B. Developmental toxicity and alteration of gene expression in zebrafish embryos exposed to PFOS. Toxicol. Appl. Pharmacol. 2008, 230, 23–32.
- Laine, M.B.; Vesamäki, J.S.; Puupponen, V.M.; Tiirola, M.; Taipale, S.J. Comparing the Ecotoxicological Effects of Perfluorooctanoic Acid (PFOA) and Perfluorohexanoic Acid (PFHxA) on Freshwater Microbial Community. Front. Environ. Sci. 2022, 10, 516.
- Cara, B.; Lies, T.; Thimo, G.; Robin, L.; Lieven, B. Bioaccumulation and trophic transfer of perfluorinated alkyl substances (PFAS) in marine biota from the Belgian North Sea: Distribution and human health risk implications. Environ. Pollut. 2022, 311, 119907.
- United Nations Industrial Development Organization (UNIDO). Preparing for HCFC Phase-Out: Fundamentals of Uses, Alternatives, Implications and Funding for Article 5 Countries; UNIDO: Vienna, Austria, 2009.
- Li, L.; Yu, N.; Wang, X.; Shi, W.; Liu, H.; Zhang, X.; Yang, L.; Pan, B.; Yu, H.; Wei, S. Comprehensive Exposure Studies of Per- and Polyfluoroalkyl Substances in the General Population: Target, Nontarget Screening, and Toxicity Prediction. Environ. Sci. Technol. 2022, 56, 14617–14626.
- Eick, S.M.; Goin, D.E.; Trowbridge, J.; Cushing, L.; Smith, S.C.; Park, J.S.; DeMicco, E.; Padula, A.M.; Woodruff, T.J.; Morello-Frosch, R. Dietary predictors of prenatal per- and poly-fluoroalkyl substances exposure. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 32–39.
- Liu, Y.; Zhang, Q.; Li, Y.; Hao, Y.; Li, J.; Zhang, L.; Wang, P.; Yin, Y.; Zhang, S.; Li, T.; et al. Occurrence of per- and polyfluoroalkyl substances (PFASs) in raw milk and feed from nine Chinese provinces and human exposure risk assessment. Chemosphere 2022, 300, 134521.
- Chen, Q.; Chou, W.C.; Lin, Z. Integration of Toxicogenomics and Physiologically Based Pharmacokinetic Modeling in Human Health Risk Assessment of Perfluorooctane Sulfonate. Environ. Sci. Technol. 2022, 56, 3623–3633.
- Amstutz, V.H.; Cengo, A.; Gehres, F.; Sijm, D.T.H.M.; Vrolijk, M.F. Investigating the cytotoxicity of per- and polyfluoroalkyl substances in HepG2 cells: A structure-activity relationship approach. Toxicology 2022, 480, 153312.
- Solan, M.E.; Senthilkumar, S.; Aquino, G.V.; Bruce, E.D.; Lavado, R. Comparative cytotoxicity of seven per- and polyfluoroalkyl substances (PFAS) in six human cell lines. Toxicology 2022, 477, 153281.
- Batzella, E.; Girardi, P.; Russo, F.; Pitter, G.; Da Re, F.; Fletcher, T.; Canova, C. Perfluoroalkyl substance mixtures and cardio-metabolic outcomes in highly exposed male workers in the Veneto Region: A mixture-based approach. Environ. Res. 2022, 212, 113225.
- Lin, C.Y.; Wang, C.; Sung, F.C.; Su, T.C. Association between serum per- and polyfluoroalkyl substances and thrombograms in young and middle-aged Taiwanese populations. Ecotoxicol. Environ. Saf. 2022, 236, 113457.
