Redox flow batteries (RFBs) have been widely recognized in the domain of large-scale energy storage due to their simple structure, long lifetime, quick response, decoupling of capacity and power, and structural simplicity. Because of the limited open circuit voltage (OCV) by hydrogen and oxygen evolution reactions, together with the relatively low solubility of active species, RFBs with aqueous electrolytes are challenging to reach high energy densities. Researchers have been trying to develop new solvent systems without water to remove the electrochemical window limitation of water and pursue higher cell potential. However, non-aqueous solvents are also hindered by some key problems, such as high viscosity and poor safety.
- large-scale energy storage
- flow battery
- non-aqueous solution
1. Introduction
2. Design and Operation Parameters of Non-Aqueous Flow Batteries
2.1. Redox Couple Design
2.2. Solvent Paring and Supporting Electrolyte Selection
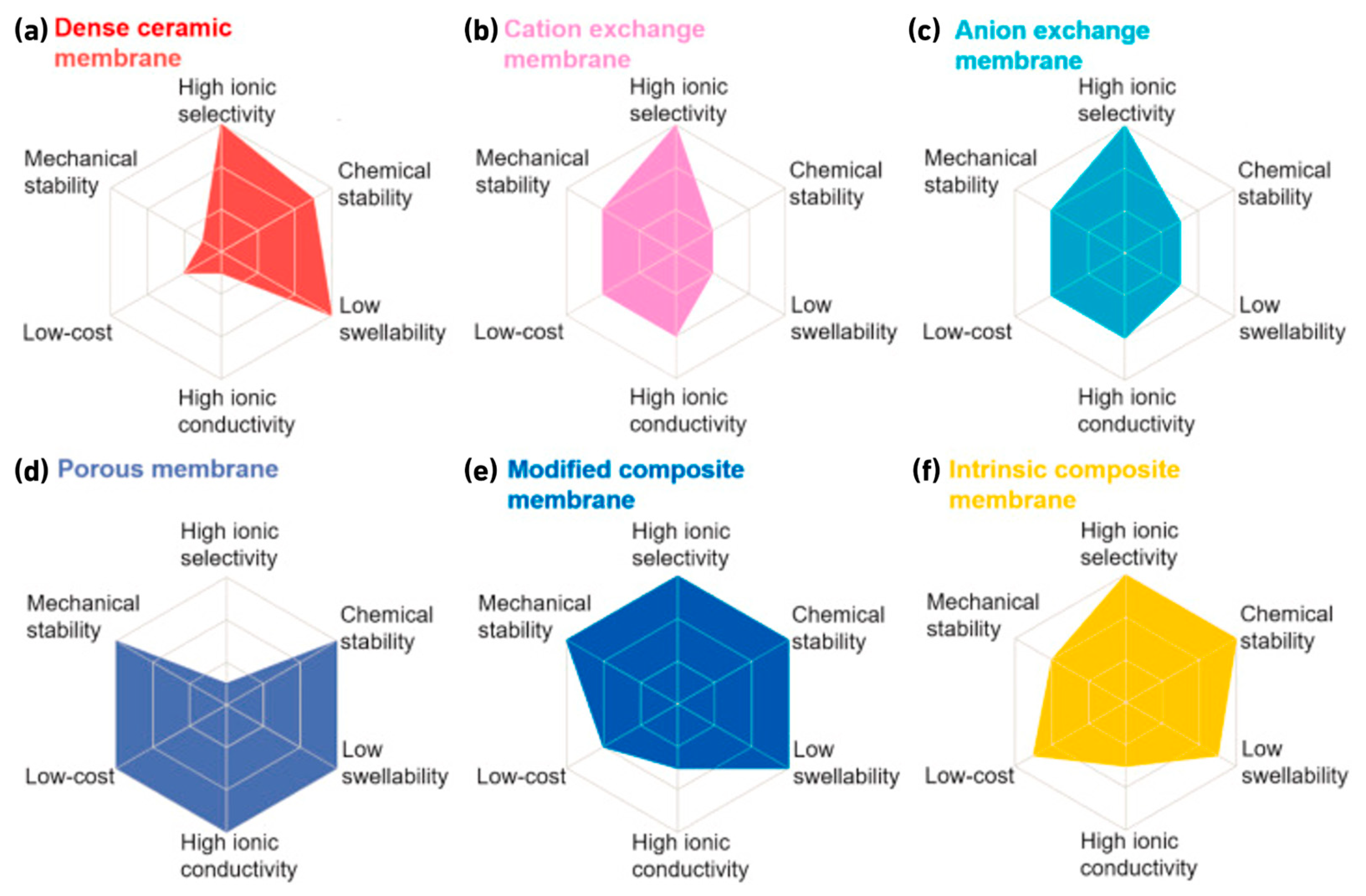
2.3. The Choice of Membrane
3. Non-Aqueous Flow Batteries with Organic Solvents
4. Non-Aqueous Flow Batteries with Ionic Liquid Solvents
This entry is adapted from the peer-reviewed paper 10.3390/batteries9040215
References
- Thaller, L.H. Electrically rechargeable redox flow cells. In Proceedings of the 9th Intersociety Energy Conversion Engineering Conference, Chicago, IL, USA, 1 January 1974; pp. 924–928.
- Zeng, Y.K.; Zhou, X.L.; An, L.; Wei, L.; Zhao, T.S. A high-performance flow-field structured iron-chromium redox flow battery. J. Power Sources 2016, 324, 738–744.
- Cho, K.T.; Ridgway, P.; Weber, A.Z.; Haussener, S.; Battaglia, V.; Srinivasan, V. High Performance Hydrogen/Bromine Redox Flow Battery for Grid-Scale Energy Storage. J. Electrochem. Soc. 2012, 159, A1806–A1815.
- Wu, M.C.; Zhao, T.S.; Jiang, H.R.; Zeng, Y.K.; Ren, Y.X. High-performance zinc bromine flow battery via improved design of electrolyte and electrode. J. Power Sources 2017, 355, 62–68.
- Li, L.; Kim, S.; Wang, W.; Vijayakumar, M.; Nie, Z.; Chen, B.; Zhang, J.; Xia, G.; Hu, J.; Graff, G.; et al. A Stable Vanadium Redox-Flow Battery with High Energy Density for Large-Scale Energy Storage. Adv. Energy Mater. 2011, 1, 394–400.
- Wills, R.G.A.; Collins, J.; Stratton-Campbell, D.; Low, C.T.J.; Pletcher, D.; Walsh, F.C. Developments in the soluble lead-acid flow battery. J. Appl. Electrochem. 2010, 40, 955–965.
- Hu, B.; DeBruler, C.; Rhodes, Z.; Liu, T.L. Long-Cycling Aqueous Organic Redox Flow Battery (AORFB) toward Sustainable and Safe Energy Storage. J. Am. Chem. Soc. 2017, 139, 1207–1214.
- Skyllas-Kazacos, M.; Chakrabarti, M.H.; Hajimolana, S.A.; Mjalli, F.S.; Saleem, M. Progress in Flow Battery Research and Development. J. Electrochem. Soc. 2011, 158, R55–R79.
- Robb, B.H.; Waters, S.E.; Marshak, M.P. Evaluating aqueous flow battery electrolytes: A coordinated approach. Dalton Trans. 2020, 49, 16047–16053.
- Huang, Y.; Gu, S.; Yan, Y.; Li, S.F.Y. Nonaqueous redox-flow batteries: Features, challenges, and prospects. Curr. Opin. Chem. Eng. 2015, 8, 105–113.
- Kowalski, J.A.; Su, L.; Milshtein, J.D.; Brushett, F.R. Recent advances in molecular engineering of redox active organic molecules for nonaqueous flow batteries. Curr. Opin. Chem. Eng. 2016, 13, 45–52.
- Ortiz-Martínez, V.M.; Gómez-Coma, L.; Pérez, G.; Ortiz, A.; Ortiz, I. The roles of ionic liquids as new electrolytes in redox flow batteries. Sep. Purif. Technol. 2020, 252, 117436.
- Hogue, R.W.; Toghill, K.E. Metal coordination complexes in nonaqueous redox flow batteries. Curr. Opin. Electrochem. 2019, 18, 37–45.
- Winsberg, J.; Hagemann, T.; Janoschka, T.; Hager, M.D.; Schubert, U.S. Redox-Flow Batteries: From Metals to Organic Redox-Active Materials. Angew. Chem.-Int. Ed. 2017, 56, 686–711.
- Bamgbopa, M.O.; Pour, N.; Shao-Horn, Y.; Almheiri, S. Systematic selection of solvent mixtures for non-aqueous redox flow batteries—Vanadium acetylacetonate as a model system. Electrochim. Acta 2017, 223, 115–123.
- Soavi, F.; Brilloni, A.; De Giorgio, F.; Poli, F. Semi-solid lithium/oxygen flow battery: An emerging, high-energy technology. Curr. Opin. Chem. Eng. 2022, 37, 100835.
- Ventosa, E. Semi-solid flow battery and redox-mediated flow battery: Two strategies to implement the use of solid electroactive materials in high-energy redox-flow batteries. Curr. Opin. Chem. Eng. 2022, 37, 100834.
- Li, L.; Yin, Y.J.; Hei, J.P.; Wan, X.J.; Li, M.L.; Cui, Y. Molecular Engineering of Aromatic Imides for Organic Secondary Batteries. Small 2021, 17, e2005752.
- Sheldon, R.A. The greening of solvents: Towards sustainable organic synthesis. Curr. Opin. Green Sustain. Chem. 2019, 18, 13–19.
- Lehmann, M.L.; Tyler, L.; Self, E.C.; Yang, G.; Nanda, J.; Saito, T. Membrane design for non-aqueous redox flow batteries: Current status and path forward. Chem 2022, 8, 1611–1636.
- Moutet, J.; Veleta, J.M.; Gianetti, T.L. Symmetric, Robust, and High-Voltage Organic Redox Flow Battery Model Based on a Helical Carbenium Ion Electrolyte. Acs Appl. Energy Mater. 2021, 4, 9–14.
- Yu, X.; Yu, W.A.; Manthiram, A. A mediator-ion nitrobenzene—Iodine nonaqueous redox flow battery with asymmetric solvents. Energy Storage Mater. 2020, 29, 266–272.
- Xu, D.; Zhang, C.; Zhen, Y.; Zhao, Y.; Li, Y. A high-rate nonaqueous organic redox flow battery. J. Power Sources 2021, 495, 229819.
- Kim, J.-H.; Kim, K.J.; Park, M.-S.; Lee, N.J.; Hwang, U.; Kim, H.; Kim, Y.-J. Development of metal-based electrodes for non-aqueous redox flow batteries. Electrochem. Commun. 2011, 13, 997–1000.
- Akhmetov, N.; Ovsyannikov, N.; Gvozdik, N.; Pogosova, M.; Ryazantsev, S.; Lipovskikh, S.; Krasnikova, I.; Stevenson, K. Composite lithium-conductive LATP+PVdF membranes: Development, optimization, and applicability for Li-TEMPO hybrid redox flow batteries. J. Membr. Sci. 2022, 643, 120002.
- Tang, L.A.; Leung, P.; Xu, Q.; Mohamed, M.R.; Dai, S.Y.; Zhu, X.; Flox, C.; Shah, A.A. Future perspective on redox flow batteries: Aqueous versus nonaqueous electrolytes. Curr. Opin. Chem. Eng. 2022, 37, 100833.
- Yuan, J.; Pan, Z.-Z.; Jin, Y.; Qiu, Q.; Zhang, C.; Zhao, Y.; Li, Y. Membranes in non-aqueous redox flow battery: A review. J. Power Sources 2021, 500, 229983.
- Armstrong, C.G.; Toghill, K.E. Stability of molecular radicals in organic non-aqueous redox flow batteries: A mini review. Electrochem. Commun. 2018, 91, 19–24.
- Chakrabarti, M.H.; Mjalli, F.S.; AlNashef, I.M.; Hashim, M.A.; Hussain, M.A.; Bahadori, L.; Low, C.T.J. Prospects of applying ionic liquids and deep eutectic solvents for renewable energy storage by means of redox flow batteries. Renew. Sustain. Energy Rev. 2014, 30, 254–270.
