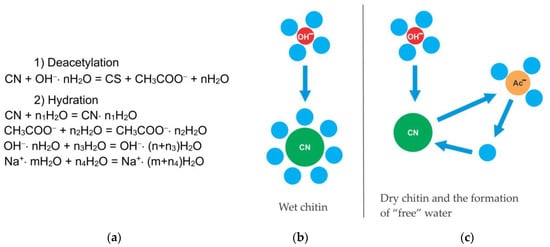
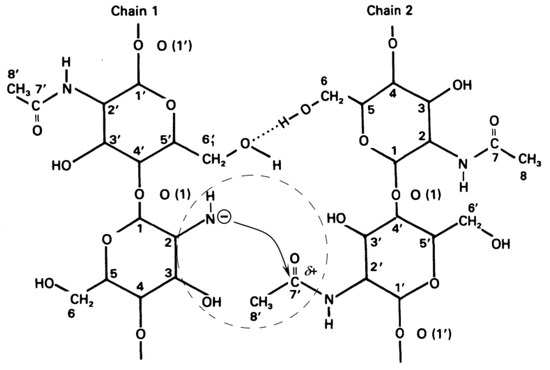
Chitosan can be obtained from chitin chemically or by using enzymatic preparations. From a chemical point of view, both acids and alkalis can be used to deacetylate chitin. However, alkaline deacetylation is used more often since glycosidic bonds are very sensitive to an acidic environment, in which they are destroyed. A mechanism for the chitin deacetylation reaction is proposed, taking into account its kinetic features in which the decisive role is assigned to the effects of hydration. It has been shown that the rate of chitin deacetylation increases with a decrease in the degree of hydration of hydroxide ions in a concentrated alkali solution. When the alkali concentration is less than the limit of complete hydration, the reaction practically does not occur. Hypotheses have been put forward to explain the decrease in the rate of the reaction in the second flat portion of the kinetic curve. The first hypothesis is the formation of “free” water, leading to the hydration of chitin molecules and a decrease in the reaction rate. The second hypothesis postulates the formation of a stable amide anion of chitosan, which prevents the nucleophilic attack of the chitin macromolecule by hydroxide ions.
- chitin
- chitosan
- deacetylation
- kinetics
- reaction mechanism
- hydration
1. Introduction
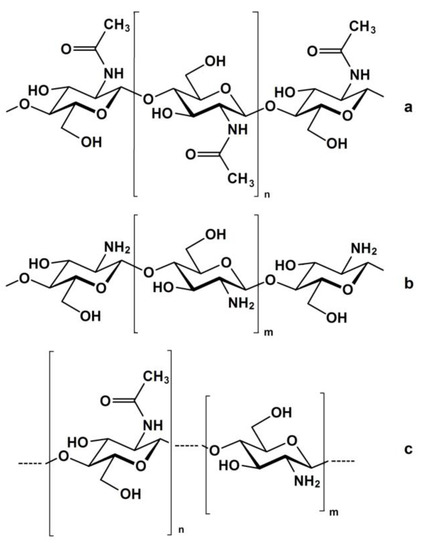
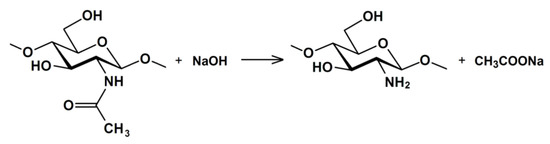
2. Kinetics of the Chitin Deacetylation Reaction
2.1. Factors Determining the Inhibition of the Deacetylation Reaction
2.2. Reversibility of the Reaction
2.3. Crystallinity of Chitin
2.4. Diffusion of Alkali
2.5. Porosity of Chitin
3. On the Mechanism of the Chitin Deacetylation Reaction
3.1. The Reaction Mechanism in the First Section of the Kinetic Curve
3.1.1. Hydration of Chitin/Chitosan and Sodium Hydroxide
3.1.2. Alkali Concentration and the Nature of the Active Particle
2.1.3. The Nature of Alkali and the Number of Hydroxide Ions Hydration
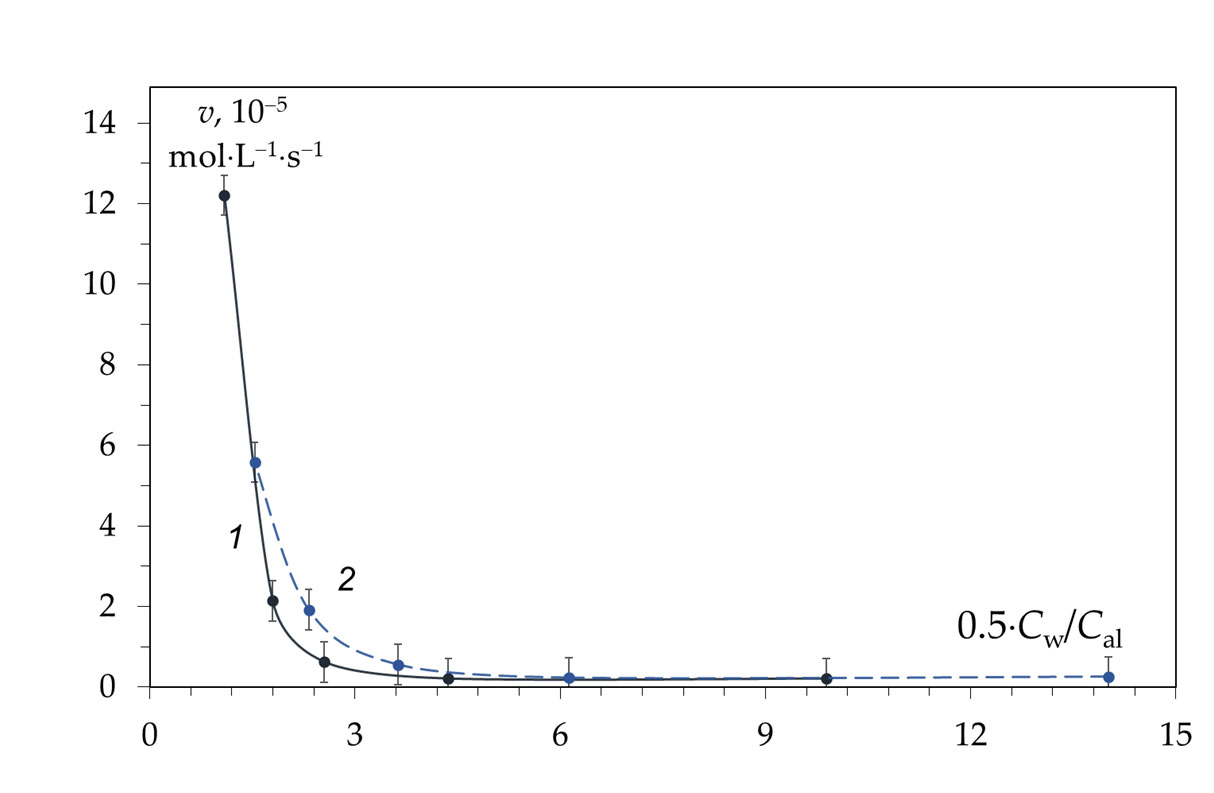
3.2. Reaction Mechanism in the Second Section of the Kinetic Curve: Inhibition of the Deacetylation Reaction
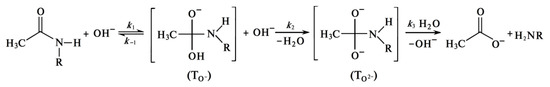
Formation of Quasi-Stable Chitosan Amide Anion
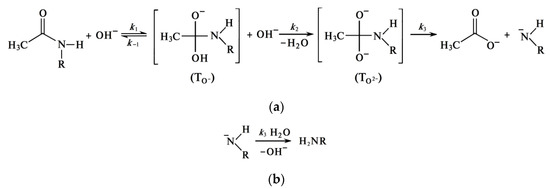
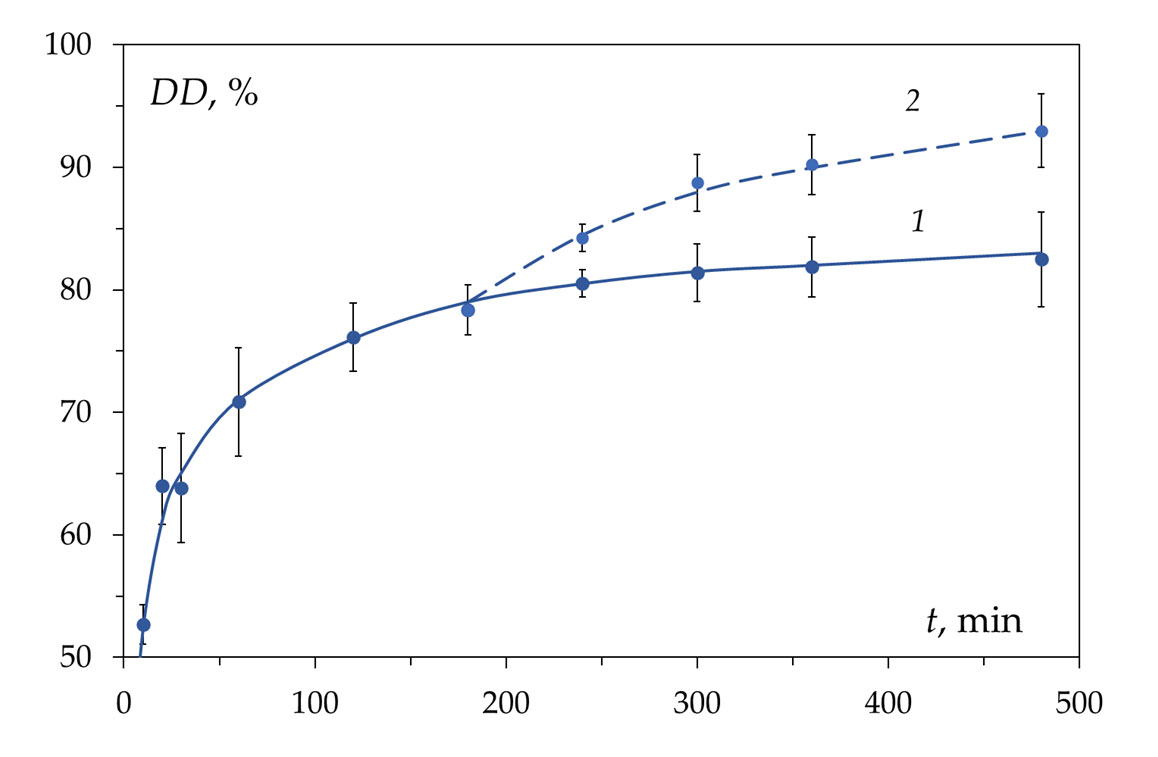
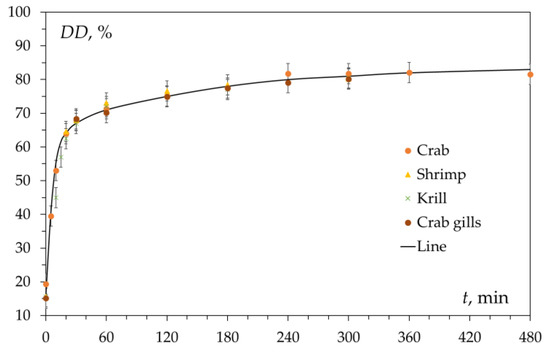
This review considers, in detail, the kinetic features of the reaction of heterogeneous deacetylation of chitin in concentrated (50% wt.) alkali solutions and analyzes the reasons for the inhibition of the reaction, which make it impossible to obtain chitosan with a limiting degree of deacetylation of 100% as a product of this reaction. The dependence of the degree of deacetylation on the reaction time has a characteristic shape consisting of two sections. The first site (or site of fast deacetylation) is characterized by a sharp increase in the degree of deacetylation within about 30 min from the start of the reaction. The second site (or the site of slow deacetylation) is characterized by a slow increase in degree of deacetylation due to a sharp decrease in the reaction rate. After 3–4 h of reaction time,
the maximum achievable value for the degree of chitosan deacetylation, equal to about (80–85)%, is reached.
A mechanism for the reaction of chitin deacetylation is proposed, which explains the features of the reaction kinetics. An analysis of publications on the decrease in the rate of the deacetylation reaction was carried out, and some new experimental results were presented, confirming the conclusions of the authors. It has been shown that water present in the reaction mixture has the greatest effect on the kinetics of chitin deacetylation, and it can lead to hydration of both chitin/chitosan macromolecules and alkali ions. Within the framework of the mechanism under consideration, a hypothesis is proposed according to which there is a dynamic equilibrium in the reaction mixture between hydrated alkali ions, chitin molecules, and the resulting acetate ions, which can shift depending on the
concentration of the reacting particles.
It has been suggested that in highly concentrated aqueous solutions of alkalis, water is almost completely in the form of hydration shells of alkali ions and, to a lesser extent, in the form of “free” water. Apparently, the rate-limiting step in the deacetylation reaction is the hydration of chitin macromolecules by water molecules, that are released during the nucleophilic substitution of acetyl radicals associated with the amino groups of chitins by hydroxyl ions. The acetate ion is the product of the deacetylation reaction. The hydration energy of the acetate ion is less than the hydration energy of the hydroxide ion, so the acetate ion is less hydrated, resulting in some “free” water being released. Under these conditions, hydration of chitin macromolecules occurs in a local region around chitin molecules, where a local high concentration of water is created after deacetylation. The concentration of alkali in the entire reaction volume remains almost constant.
Establishing the mechanism of the chitin deacetylation reaction is of practical importance for the development of technologies for the production of chitosan, and it also makes a theoretical contribution to the understanding of the role of the solvent and hydration processes when considering the kinetics of heterogeneous reactions.
This entry is adapted from the peer-reviewed paper 10.3390/polym15071729
References
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226.
- Younes, I.; Rinaudo, M. Chitin and Chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174.
- Hajji, S.; Younes, I.; Ghorbel-Bellaaj, O.; Hajji, R.; Rinaudo, M.; Nasri, M.; Jellouli, K. Structural differences between chitin and chitosan extracted from three different marine sources. Int. J. Biol. Macromol. 2014, 65, 298–306.
- Hasan, S.; Boddu, V.M.; Viswanath, D.S.; Ghost, T.K. Chitin and Chitosan: Science and Engineering; Springer: Cham, Switzerland, 2022; p. 421.
- Hou, F.; Gong, Z.; Jia, F.; Cui, W.; Song, S.; Zhang, J.; Wang, Y.; Wang, W. Insights into the relationships of modifying methods, structure, functional properties and applications of chitin: A review. Food Chem. 2023, 409, 135336.
- Wei, A.; Fu, J.; Guo, F. Mechanical properties of chitin polymorphs: A computational study. J. Mater. Sci. 2021, 56, 12048–12058.
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021, 159, 110709.
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186.
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27.
- Muzzarelli, R.A.A. The discovery of chitin. In Chitosan in Pharmacy and Chemistry; Muzzarelli, R.A.A., Muzzarelli, C., Eds.; Atec: Grottammare, Italy, 2002; pp. 1–8.
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632.
- Akpan, E.I.; Gbenebor, O.P.; Adeosun, S.O.; Cletus, O. Chapter 5—Solubility, degree of acetylation, and istribution of acetyl groups in chitosan. In Handbook of Chitin and Chitosan: Volume 1: Preparation and Properties; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 131–164.
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194.
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Chitin and chitosan: History, fundamentals and innovations. In Sustainable Agriculture Reviews 35: Chitin and Chitosan: History, Fundamentals and Innovations; Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2019; Volume 35, pp. 49–123.
- Percot, A.; Chaussard, G.; Sorlier, P.; Schatz, C.; Montembault, A.; Viton, C. Overall consideration on the evolution of the study of chitosan properties. In Advances in Chitin Science; Boucher, I., Jamieson, K., Retnakaran, A., Eds.; European Chitin Society: Montreal, QC, Canada, 2004; Volume 7, pp. 1–6.
- Xu, D.; Aihemaiti, Z.; Cao, Y.; Teng, C.; Li, X. Physicochemical stability, microrheological properties and microstructure of lutein emulsions stabilized by multilayer membranes consisting of whey protein isolate, flaxseed gum and chitosan. Food Chem. 2016, 202, 156–164.
- Abere, D.V.; Ojo, S.A.; Paredes-Epinosa, M.B.; Hakami, A. Derivation of composites of chitosan-nanoparticles from crustaceans source for nanomedicine: A mini review. Adv. Biomed. Eng. 2022, 4, 100058.
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.; Ahmed, S. A review on chitosan and chitosan-based bionanocomposites: Promising material for combatting global issues and its applications. Int. J. Biol. Macromol. 2021, 85, 832–848.
- Sami El-banna, F.; Mahfouz, M.E.; Leporatti, S.; El-Kemary, M.A.; Hanafy, A.N.N. Chitosan as a natural copolymer with unique properties for the development of hydrogels. Appl. Sci. 2019, 9, 2193.
- Pellá, M.C.G.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245.
- Do, N.H.N.; Truong, Q.T.; Le, P.K.; Ha, A.C. Recent developments in chitosan hydrogels carrying natural bioactive compounds. Carbohydr. Polym. 2022, 294, 119726.
- Xu, J.; Zhang, M.; Du, W.; Zhao, J.; Ling, G.; Zhang, P. Chitosan-based high-strength supramolecular hydrogels for 3D bioprinting. Int. J. Biol. Macromol. 2022, 219, 545–557.
- Meng, Q.; Zhong, S.; Wang, J.; Gao, Y.; Cui, X. Advances in chitosan-based microcapsules and their applications. Carbohydr. Polym. 2023, 300, 120265.
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825.
- Chen, S.; Tian, H.; Mao, J.; Ma, F.; Zhang, M.; Chen, F.; Yang, P. Preparation and application of chitosan-based medical electrospun nanofibers. Int. J. Biol. Macromol. 2023, 226, 410–422.
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosanin food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692.
- Rebello, S.; Sali, S.; Jisha, M.S.; Reshmy, R.; Pugazhendhi, A.; Madhavan, A.; Binod, P.; Awasthi, M.K.; Pandey, A.; Sindhu, R. Chitosan a versatile adsorbent in environmental remediation in the era of circular economy- a mini review. Sustain. Chem. Pharm. 2023, 32, 101004.
- Issahaku, I.; Tetteh, I.K.; Tetteh, A.Y. Chitosan and chitosan derivatives: Recent advancements in production and applications in environmental remediation. Environ. Adv. 2023, 11, 100351.
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594.
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Fundamentals of Chitosan for Biomedical Applications. In Chitosan Based Biomaterials, Volume 1: Fundamentals; Jennings, J.A., Bumgardner, J.D., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 3–30.
- Kedir, W.M.; Abdi, G.F.; Goro, M.M.; Tolesa, L.D. Pharmaceutical and drug delivery applications of chitosan biopolymer and its modified nanocomposite: A review. Heliyon 2022, 8, e10196.
- Baharlouei, P.; Rahman, A. Chitin and chitosan: Prospective biomedical applications in drug delivery, cancer treatment, and wound healing. Mar. Drugs 2022, 20, 460.
- Sharma, P.P.; Bhardwaj, S.; Sethi, A.; Goel, V.K.; Grishina, M.; Poonam; Rathi, B. Chitosan based architectures as biomedical carriers. Carbohydr. Res. 2022, 522, 108703.
- Riseh, R.S.; Vatankhah, M.; Hassanisaadi, M.; Kennedy, J.F. Chitosan-based nanocomposites as coatings and packaging materials for the postharvest improvement of agricultural product: A review. Carbohydr. Polym. 2023, 309, 120666.
- Fan, Z.; Wang, L.; Qin, Y.; Li, P. Activity of chitin/chitosan/chitosan oligosaccharide against plant pathogenic nematodes and potential modes of application in agriculture: A review. Carbohydr. Polym. 2023, 306, 120592.
- Xu, K.; Li, L.; Huang, Z.; Tian, Z.; Li, H. Efficient adsorption of heavy metals from wastewater on nanocomposite beads prepared by chitosan and paper sludge. Sci. Total Environ. 2022, 846, 157399.
- Gal, M.R.; Rahmaninia, M.; Hubbe, M.A. A comprehensive review of chitosan applications in paper science and technologies. Carbohydr. Polym. 2023, 309, 120665.
- Van den Broek, L.A.M.; Knoop, R.J.I.; Kappen, F.H.J.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242.
- Elwakeel, K.Z. Environmental application of chitosan resins for the treatment of water and wastewater: A Review. J. Dispers. Sci. Technol. 2010, 31, 273–288.
- Choi, C.; Nam, J.-P.; Nah, J.-W. Application of chitosan and chitosan derivatives as biomaterials. J. Ind. Eng. Chem. 2016, 33, 1–10.
- Chauhan, S.; Thakur, A. Chitosan-based biosensors-A comprehensive Review. Mater. Today Proc. 2023.
- Philibert, T.; Lee, B.H.; Fabien, N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337.
- Ahlafi, H.; Moussout, H.; Boukhlifi, F.; Echetna, M.; Bennani, M.N.; Slimane, S.M. Kinetics of N-deacetylation of chitin extracted from shrimp shells collected from coastal area of Morocco. Mediterr. J. Chem. 2013, 2, 503–513.
- Novikov, V.Y.; Konovalova, I.N.; Dolgopyatova, N.V. The mechanism of chitin and chitosan deacetylation during long-term alkaline treatment. Appl. Biochem. Microbiol. 2022, 58, 309–314.
- Novikov, V.Y. The general relationships of chitin and chitosan chemical hydrolysis. In Advances in Chitin Science; Struszczyk, H., Domard, A., Peter, M.G., Pospieszny, H., Eds.; Publisher Institute of Plant Protection: Poznan, Poland, 2005; Volume 8, pp. 109–113.
- Chebotok, E.N.; Novikov, V.Y.; Konovalova, I.N. Kinetics of base deacetylation of chitin and chitosan as influenced by their crystallinity. Russ. J. Appl. Chem. 2007, 80, 1753–1758.
- Dolgopiatova, N.V.; Kuchina, Y.A.; Dyakina, T.N.; Volkova, T. Effect of heterogeneous deacetylation on the properties of northern shrimp chitin and chitosan. KnE Life Sci. 2020, 5, 315–324.
- Novikov, V.Y.; Rysakova, K.S.; Shumskaya, N.V.; Mukhortova, A.M.; Kesarev, K.A. King crab gills as a new source of chitin/chitosan and protein hydrolysates. Int. J. Biol. Macromol. 2023, 232, 123346.
- Narudin, N.A.H.; Rosman, N.A.; Shahrin, E.W.; Sofyan, N.; Mahadi, A.H.; Kusrini, E.; Hobley, J.; Usman, A. Extraction, characterization, and kinetics of N-deacetylation of chitin obtained from mud crab shells. Polym. Polym. Compos. 2022, 30, 09673911221109611.
- Liu, T.G.; Li, B.; Huang, W.; Lv, B.; Chen, J.; Zhang, J.X.; Zhu, L.P. Effects and kinetics of a novel temperature cycling treatment on the N-deacetylation of chitin in alkaline solution. Carbohydr. Polym. 2009, 77, 110–117.
- Bradić, B.; Bajec, D.; Pohar, A.; Novak, U.; Likozar, B. A reaction–diffusion kinetic model for the heterogeneous N-deacetylation step in chitin material conversion to chitosan in catalytic alkaline solutions. React. Chem. Eng. 2018, 3, 920–929.
- De Moura, C.M.; de Moura, J.M.; Soares, N.M.; de Almeida Pinto, L.A. Evaluation of molar weight and deacetylation degree of chitosan during chitin deacetylation reaction: Used to produce biofilm. Chem. Eng. Process. 2011, 50, 351–355.
- Jiang, C.J.; Xu, M.Q. Kinetics of heterogeneous deacetylation of β-Chitin. Chem. Eng. Technol. 2006, 29, 511–516.
- De Souza, J.R.; Giudici, R. Effect of diffusional limitations on the kinetics of deacetylation of chitin/chitosan. Carbohydr. Polym. 2021, 254, 117278.
- Kurita, K.; Sannan, T.; Iwakura, Y. Studies on chitin, 4. Evidence for formation of block and random copolymers of N-acetyl-D-glucosamine and D-glucosamine by hetero- and homogeneous hydrolysis. Makromol. Chem. 1977, 178, 3197–3202.
- Yaghobi, N.; Mirzadeh, H. Enhancement of chitin’s degree of deacetylation by multistage alkali treatments. Iran. Polym. J. 2004, 13, 131–136.
- Varum, K.M.; Anthonsen, M.W.; Grasdalen, H.; Smidsrod, O. Determination of the degree of N-acetylation and the distribution of N-acetyl groups in partially N-deacetylated chitins (chitosans) by high-field nmr spectroscopy. Carbohydr. Res. 1991, 211, 17–23.
- Varum, K.M.; Anthonsen, M.W.; Grasdalen, H.; Smidsrod, O. 13C-N.m.r. studies of the acetylation sequences in partially N-deacetylated chitins (chitosans). Carbohydr. Res. 1991, 217, 19–27.
- Lamarque, G.; Cretenet, M.; Lucas, J.; Crepet, A.; Viton, C.; Domard, A. Optimization of α- and β-chitin heterogeneous de-N-acetylation from a multi-step process: New route of de-N-acetylation by means of freeze-pump-thaw cycles. In Advances in Chitin Science; Boucher, I., Jamieson, K., Retnakaran, A., Eds.; European Chitin Society: Montreal, QC, Canada, 2004; Volume 7, pp. 66–73.
- Lamarque, G.; Cretenet, M.; Viton, C.; Domard, A. New route of deacetylation of alpha- and beta-chitins by means of freeze-pump out-thaw cycles. Biomacromolecules 2005, 6, 1380–1388.
- Sikorski, P.; Hori, R.; Wada, M. Revisit of alpha-chitin crystal structure using high resolution X-ray diffraction data. Biomacromolecules 2009, 10, 1100–1105.
- Lamarque, G.; Viton, C.; Domard, A. Comparative study of the second and third heterogeneous deacetylations of alpha- and beta-chitins in a multistep process. Biomacromolecules 2004, 5, 1899–1907.
- Lavertu, M.; Darras, V.; Buschmann, M.D. Kinetics and efficiency of chitosan reacetylation. Carbohydr. Polym. 2012, 87, 1192–1198.
- Methacanon, P.; Prasitsilp, M.; Pothsree, T.; Pattaraarchachai, J. Heterogeneous N-deacetylation of squid chitin in alkaline solution. Carbohydr. Polym. 2003, 52, 119–123.
- Chebotok, E.N.; Novikov, V.Y.; Konovalova, I.N. Depolymerization of chitin and chitosan in the course of base deacetylation. Russ. J. Appl. Chem. 2006, 79, 1162–1166.
- Nikolov, S.; Fabritius, H.; Petrov, M.; Friak, M.; Lymperakis, L.; Sachs, C.; Raabe, D.; Neugebauer, J. Robustness and optimal use of design principles of arthropod exoskeletons studied by ab initio-based multiscale simulations: Multiscale mechanics. J. Mech. Behav. Biomed. Mater. 2011, 4, 129–145.
- Novikov, V.; Derkach, S.; Konovalova, I. Chitosan technology from crustacean shells of the northern seas. KnE Life Sci. 2020, 5, 65–74.
- Khong, T.T.; Aachmann, F.L.; Vrum, K.M. Kinetics of de-N-acetylation of the chitin disaccharide in aqueous sodium hydroxide solution. Carbohydr. Res. 2012, 352, 82–87.
- Hummer, G.; Pratt, L.R.; Garcia, A.E. Free energy of ionic hydration. J. Phys. Chem. 1996, 100, 1206–1215.
- Pearson, R.G. Ionization potentials and electron affinities in aqueous solution. J. Am. Chem. Soc. 1986, 108, 6109–6114.
- Chen, C.H.; Wang, F.Y.; Ou, Z.P. Deacetylation of β-chitin. I. Influence of the deacetylation conditions. J. Appl. Polym. Sci. 2004, 93, 2416–2422.
- Jaworska, M.M. Kinetics of enzymatic deacetylation of chitosan. Cellulose 2012, 19, 363–369.
- Tsaih, M.L.; Chen, R.H. The effect of reaction time and temperature during heterogenous alkali deacetylation on degree of deacetylation and molecular weight of resulting chitosan. J. Appl. Polym. Sci. 2003, 88, 2917–2923.
- Khan, M.N. Experimental versus theoretical evidence for the rate-limiting steps in uncatalyzed and H+- and HO−-catalyzed hydrolysis of the amide bond. Int. J. Chem. Kinet. 2009, 41, 599–611.
- Carroll, F.A. Perspectives on Structure and Mechanism in Organic Chemistry, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; p. 966.
- Brown, R.S. Studies in amide hydrolysis: The acid, base, and water reactions. In The Amide Linkage. Structural Significance in Chemistry, Biochemistry, and Materials Science; Greenberg, A., Breneman, C.M., Liebman, J.F., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 85–114.
- Challis, B.C.; Challis, J.A. Chapter 13. Reactions of the carboxamide group. In The Chemistry of Amides; Zabicky, J., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1970; pp. 731–857.
- Xiong, Y.; Zhan, C.-G. Theoretical studies of the transition-state structures and free energy barriers for base-catalyzed hydrolysis of amides. J. Phys. Chem. A 2006, 110, 12644–12652.
- Roberts, G.A.F. Chitin Chemistry; The Macmillan Press Ltd.: Basingstoke, UK; London, UK, 1992; p. 368.
- Muzzarelli, R.A.A. Chitin nanostructures in living organisms. In Chitin. Formation and Diagenesis (Topics in Geobiology); Gupta, N.S., Ed.; Springer: Dordrecht, The Netherlands, 2011; Volume 34, pp. 1–34.
