1. Why LTLV Occurs?
At the technical level, the quality of immunoassays is determined by two key elements: raw materials and production processes. It is estimated that 70% of the immunoassay’s performance is attributed to the raw materials, while the rest 30% is ascribed to the production process (such as buffer recipes, reagents formulation, etc.).The production process guarantees the lower limit of kit quality and reproducibility, while raw materials provide the foundation for the sensitivity and specificity of IVD kits, thereby forming the upper limit of kit quality, akin to the icing on the cake. To pinpoint the causes and origins of lot-to-lot variance (LTLV), it is necessary to explore both of these aspects in detail. Additionally, the instability of the analyte epitope, to some degree, also contributes to LTLV.
2. Quality Fluctuation of Raw Materials
An immunoassay typically comprises various components, such as solid phases (e.g., magnetic particles, microtiter plates), antibodies, antibody conjugates, antigens, antigen conjugates, calibrators, kit controls, and assay buffers or diluents. Therefore, LTLV could potentially arise from any of these aforementioned constituents. Previous experience has shown that fluctuations and instability in immunoassays are largely related to the quality of raw materials, which are inherently biologics that are difficult to regulate, such as antibodies sourced from hybridoma. Moreover, other external materials, such as the master calibrator and lot-to-lot QC panel, may also impact LTLV.
2.1. Antigens
Antigens are molecules that trigger the immune system to generate antibodies against them, and they can be comprised of various substances such as proteins, polysaccharides, lipids, chemicals, or nucleic acids. In the context of immunoassays, the quality of antigen raw materials is critical, but a uniform standard for performance evaluation is currently lacking. Generally, antigen activity, purity, batch-to-batch consistency, and stability are the key evaluation criteria. Antigens are typically provided as clear and homogeneous liquids or lyophilized white powders that are free of contaminants, turbidity, and particulates. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) is a standard method for assessing antigen purity and molecular weight, often followed by staining with Coomassie brilliant blue or silver. Additionally, a size exclusion column combined with high-performance liquid chromatography (SEC-HPLC) can also be used to determine purity and molecular weight. If the purity of the antigen is compromised, the efficiency of labeling may be impacted, leading to reduced specificity, signal, and increased background. Some antigens are unstable and require specific storage buffers that include protein stabilizers, such as bovine serum albumin (BSA)
[1], urea, and glycerol
[2]. Most antigens can be stored at −20 °C or −70 °C, and they should be frozen after being diluted and aliquoted. For synthetic peptides used as calibrators or quality controls, it is crucial to note that different batches of peptides may have varying amounts of the target peptide content due to truncated by-products from the synthesis process, although the gross peptide content may still be the same.
2.2. Enzymes
Horseradish peroxidases (HRP) and alkaline phosphatase (ALP) are enzymes that are commonly employed in IVD reagents. Enzymes are one of the few substances that can be measured using “activity units” rather than purity and mass. Horseradish contains at least seven isozymes of HRP, with isozyme C exhibiting the highest catalytic activity, accounting for up to 50% of the peroxidase content of horseradish
[3]. Thus, it is not always necessary to utilize the most purified form of HRP, as this may lead to elevated background noise in certain assays. HRP and ALP are typically obtained via extraction from native materials, such as horseradish root and calf small intestine, respectively, and are then subjected to complex purification processes to obtain the required enzymes
[3]. Given the reasonable cost limitations, a purity of 90–95% is typically deemed acceptable with current purification techniques. However, it is difficult to ascertain the number of biologically active enzymes with correct structures within this 5% impurity, and the presence of any interfering or inhibitory ingredients is sometimes unknown. As such, accurately controlling enzyme quality can be challenging. Although enzyme purity is consistent across batches, there are often notable differences in enzymatic activity. Thus, care must be taken when selecting enzyme manufacturers who claim to have improved production processes and have passed quality inspections, as such modifications may also affect other biological activities of the enzyme, ultimately impacting the expected assay performance.
2.3. Antibodies
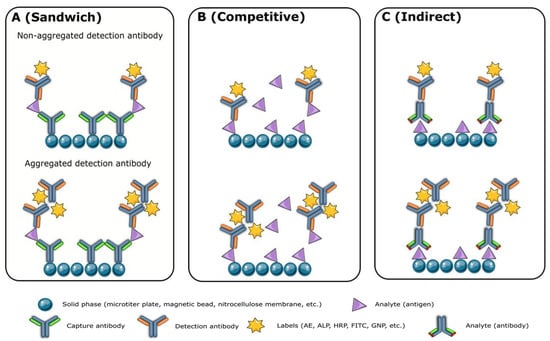
Monoclonal antibodies of high quality are crucial for the production of reliable reagents. However, modifications and labeling processes may affect the performance of the final product. Unfortunately, there is no standardized method to measure antibody quality, although activity, concentration, affinity, homogeneity, specificity, purity, and stability are commonly evaluated. Aggregation of high-concentration antibodies, particularly IgG3
[4][5][6], is a major issue that can be detected and separated using SEC-HPLC. Aggregates, fragments, and unpaired chains of antibodies can lead to high background and signal leap, causing overestimated analyte concentrations in the sandwich (
Figure 1A) and indirect immunoassays (
Figure 1C) and underestimated levels in competitive immunoassays (
Figure 1B). Antibody labeling efficiency is related to purity, and impurity proteins can negatively impact the assay’s specificity, signal, and background. Antibody purity analysis, using methods such as SDS-PAGE, SEC-HPLC, and capillary electrophoresis sodium dodecyl sulfate gel electrophoresis (CE-SDS), is critical for successful immunoassay development. The cell culture process and antibody purification process largely determine antibody purity, and the use of a serum-free medium can remove impurities brought by fetal bovine serum.
Figure 1. The impact of antibody aggregates on the sandwich (A), competitive (B), and indirect immunoassays (C). AE: acridinium ester; ALP: alkaline phosphatase; FITC: fluorescein-5-isothiocyanate; GNP: gold nanoparticles; HRP: horse radish peroxidase.
2.4. Antibody and Antigen Conjugates (Enzyme, Biotin, Fluor, etc.)
Some commercial protein conjugation kits can rapidly produce conjugates within an hour, which is advantageous in terms of saving time and resources. However, despite their seemingly straightforward process, “mix and use” conjugation products are known to be problematic due to the inefficiency of conjugation chemistry and the absence of a purification step. As a consequence, unreacted biomolecules and excess labels, such as fluorophores, biotins, and enzymes, may remain in the reaction mixture.
2.5. Kit Controls and Calibrators
Kit controls are typically produced using the same process and constituents as the kit calibrators, leading to a lack of independence that renders the kit control unsuitable for monitoring the integrity of the calibrator. In the event of instability arising from the control/calibrator materials or process, the kit control will shift to the same degree as the kit calibrator due to their identical composition, potentially giving a false sense of security regarding test performance. Any degradation of these materials would occur in both the controls and the calibrators, making it appear as though the test was still functional while it had actually shifted. In essence, kit controls serve as an additional set of calibrators or “pseudo controls”. For instance, our laboratory observed that certain laboratory-developed tests (LDTs) had acceptable shelf-life in the kit controls and calibrators, which were created using the same synthetic peptides, whereas the serum QC-panel failed to meet the criteria (data not yet published).
The use of pooled patient serum as kit controls by many manufacturers is a widely employed practice, but it raises certain overlooked issues, particularly concerning the consistency of manufacture. The lack of a standardized procedure for producing patient pool controls leads to significant variations in the manufacturing process, which, in turn, affects the stability and consistency of the product from one vial to another. The validation of the manufacturing process, dispensing, stability, and vial-to-vial homogeneity, among other factors, is typically not carried out for pooled patient biofluid controls.
2.6. Buffer/Ultrapure Water
The assay buffer plays a crucial role in immunoassays as it serves as a diluent for various components, including calibrators, samples, antibodies, and antigens. It contains a mixture of proteins, salts, detergents, coloring agents, preservatives, and ultrapure or de-ionized water, which is carefully formulated to block non-specific interactions and maintain a stable pH environment for antibody and antigen immunoreaction. The optimization of assay buffers aims to minimize background signals and achieve high assay sensitivity. The conductivity of the water used in kit production can vary (e.g., 0.05–0.8 μS/cm) across different locations and impact the performance of the immunoassay.
2.7. Others (Containers, Microtiter Plates, etc.)
In the early stages of assay development, it is important to pay attention to small consumables that may pose unexpected challenges during product implementation. The quality and cleanliness of tubes containing reagents must be assessed thoroughly to ensure their usability and compliance with cleanliness standards. The presence of residual pollutants in tubes may vary across different products, underscoring the need to verify the qualification of tube materials through experimental design. A limited number of sampling inspections are insufficient to meet evaluation requirements, and more reliable results can be obtained by conducting multiple batch and sampling tests.
3. Deviation in the Production Process
The quality of IVD products is largely influenced by the production process of immunoassays. The 4P1E (people, products, procedures, premises, and equipment) or 4M1E (men, materials, methods, machines, and environment) are the five crucial elements that significantly impact the quality of the final products. Each element encompasses various factors, such as the skills and experience of the staff, the quality of raw materials, the precision of the production process, the suitability of the production facility, and the efficiency of the production equipment. Thorough attention to these factors is crucial for ensuring the reproducibility, reliability, and accuracy of immunoassays.
3.1. People/Men
In the production of immunoassays, the quality of reagents may be compromised due to various factors during production processes, such as buffer preparation, conjugation, coating, blocking, drying, lyophilization, etc. Even if the same operator performs these tasks at different time points, it is challenging to ensure the reproducibility of the produced reagents. The introduction of personnel changes and the variability of operation tasks further exacerbate the uncertainty associated with the production process, leading to potential compromises in the quality of the reagents.
3.2. Products/Materials
The task of controlling incoming materials can prove challenging, especially when introducing new batches of antigens and antibodies or modifying key material components, even from different suppliers. As such, a comprehensive verification process must be conducted akin to that of producing new products. Even when replacing microtiter plates, magnetic beads, nitrocellulose membranes, and other consumables, resulting product quality may vary. Notably, in 2016, Life Technologies A/S discontinued the use of diglyme in the manufacturing of Dynabeads due to its classification as a reproductive toxin and listing in Annex XIV of the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) Regulation by the European Chemical Agency
[7]. Consequently, the European Union market cannot use diglyme, and customers such as Roche Diagnostics GmbH had to assess alternative solvents coated on new magnetic beads
[8]. However, some IVD assays yielded unsatisfactory reproducibility results, leading Roche to apply for authorization for the future use of diglyme in the EU
[9].
3.3. Procedures/Methods
Buffer preparation serves as a pertinent example of general preparation procedures, and although it is commonplace in most laboratories, it requires careful execution to avoid errors and is often a time-consuming and labor-intensive process. Buffer preparation entails several steps, including weighing the components, dissolving and mixing the reagents in a suitable container, adjusting and checking pH, and adjusting the final volume. During the mixing process, substance parameters such as density and viscosity bear great significance as they determine the energy requirements, miscibility, dispersing degree, and mixture stability. However, the complexity of buffer preparation has raised several questions that must be addressed, such as the optimal mixing duration and which device to use for different volumes. In general, for a one-liter solution, mixing should last for at least an hour, with the choice of the device depending on the buffer volume, where a magnetic stirrer is suitable for less than five liters and an overhead stirrer for volumes exceeding ten liters.
3.4. Equipment and Premises/Machines and Environment
The correlation between weather conditions and the performance of certain equipment, such as dehumidifiers, has been identified as a challenging factor in ensuring consistent drying quality. In particular, during rainy, foggy, cloudy, and sunny days, the same level of drying quality cannot be guaranteed due to the impact of these weather conditions. For strip test manufacturers, dispensing antibodies on nitrocellulose membranes during high humidity (>60%) can lead to wider T and C lines, resulting in smear issues. Conversely, low humidity (<40%) can cause uneven scribing and satellite points on the membrane due to strong electrostatic interaction. To overcome these issues, a recommended best practice is to balance the equipment and nitrocellulose membranes for several hours in an environment with a humidity level of 50% before dispensing. This approach is deemed necessary to ensure consistent and reliable performance of the equipment and membranes, regardless of the prevailing weather conditions.
4. Unstable Analyte Epitope
4.1. Proteinase Cleavage of the Analyte
The structural and stability properties of numerous analytes remain largely unexplored, leading to potential degradation by proteinases present in blood circulation. Peptidyl Peptidase IV (DPP IV), Neprilysin (NEP), Corin, and the Insulin-degrading Enzyme (IDE) have been identified as some of the proteinases capable of cleaving specific amino bonds, including Pro-Lys, Met-Val, Gly-Cys, Arg-Ile, Lys-Met, Leu-Arg, and Arg-Arg
[10]. Such cleavage events generate issues with LTLV in immunoassays as the analyte level in QC panels may gradually decrease over time. The proteolytic degradation of brain natriuretic peptide (BNP) into various fragments by enzymes such as DPP IV
[11], NEP
[12][13], and IDE
[14], as well as the hydrolysis of cTnI
[15][16] at the N- and C-terminals, exemplify the impact of these proteinases on analyte stability. This proteolytic degradation is not limited to in vivo conditions, as it can also occur in vitro in blood samples. The existence of various truncated forms of BNP and cTnI poses significant challenges to the accuracy of detection, highlighting the need for further research to address LTLV issues in immunoassays.
4.2. Glycosylation
Glycosylation modifications are estimated to occur in nearly 50% of proteins and have been identified as a means of enhancing analyte stability against cleavage. However, the presence of glycosylation on the epitope of interest may also interfere with antibody and antigen interactions, leading to an underestimation of analyte concentrations
[17]. The lack of international standards for some glycosylated analytes has resulted in the use of homemade master calibrators in many commercial immunoassays. Typically, these calibrators are nonglycosylated, but most antibodies used in such assays are directed against potential glycosylation sites on antigens that are nonglycosylated. This discrepancy can lead to a commutability problem between calibrators and clinical samples
[18]. The heterogeneous glycosylation patterns observed on some analytes from different human specimens
[19][20] may also contribute to LTLV. Therefore, the impact of glycosylation on the performance of immunoassays requires further investigation to establish standardized protocols for calibrator preparation and antibody production that account for potential glycosylation sites. Such efforts will ultimately improve the accuracy and reliability of glycosylated analyte measurements in clinical settings.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13111835