Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Microalgae have been a source of useful compounds mainly used as food and dietary supplements. They have been used as a source of metabolites that can participate in the synthesis of several nanoparticles through inexpensive and environmentally friendly routes alternative to chemical synthesis.
- microalgae
- nanoparticles
- synthesis
- biomedical
- anticancer
- antimicrobial
1. Introduction
The increasing human population and life expectancy are causing a change in the leading cause of death, such as heart conditions, cancer, or pulmonary diseases [1]. The shift is pushing the healthcare system to find new solutions to these problems; however, hospitals can be a source of nosocomial infections which are especially dangerous for immunocompromised patients. Another major issue is microbial antibiotic resistance due to antibiotics abuse which can lead to the emergence of life-threatening diseases [2].
New promising solutions are introduced by recent developments in nanotechnology which are focused on the manipulation of matter having a characteristic size lower than 100 nm in at least one dimension. The prevailing quantum effect at such a scale can give rise to multiple applications of the products. The prepared materials at the nanoscale might have different features than their bulk equivalents which show the potential for obtaining various properties even within the same element. Several methods are used to synthesize nanoparticles (NPs) such as physical, chemical, or biological routes. Among them, great attention is being paid to biological synthesis due to its low toxicity and biocompatibility, which are crucial for biomedical applications.
One group of organisms used for biological synthesis is microalgae due to their rapid increase in biomass, the independence on arable land, and the abundance of valuable metabolites [3]. Moreover, microalgae can be cultivated also in the wastewater independently from seasonal breaks which is an important economical aspect [4]. The bioactive substances derived from secondary metabolism such as proteins, polysaccharides, lipids, vitamins, and pigments have displayed their great potential for many applications [5] The identified compounds are mainly used for their nutritional value; however, they can participate in the synthesis of various NPs used for biomedical applications.
2. Biological Synthesis
2.1. Microalgal Metabolites
Microalgae are single-celled, photosynthetic organisms found in both marine and freshwater ecosystems. The classification of these organisms is based on the properties such as pigmentation, photosynthetic membrane organization, chemical nature of the photosynthetic storage products, or morphological features [6]. The groups are polyphyletic and highly diverse, with both procaryotic and eucaryotic organisms. The most abundant microalgae are Cyanophyceae (blue-green algae), Bacillariophyceae (including the diatoms), and Chlorophyceae (green algae), with 50,000 estimated existing species, out of which 30,000 species were investigated [6]. Microalgae produce a variety of substances including proteins, carbohydrates, lipids, nucleic acids, vitamins, and minerals [8,9,10]. The cellular content of each group varies depending on the specific strain and their physiological reactions to biotic and/or abiotic factors such as light intensity, photoperiod, temperature, medium composition, and growth phase [11,12]. The typical compounds reported so far participating in the synthesis of nanoparticles are proteins, carbohydrates, and lipids.
The main mechanism involved in the biosynthesis of NPs deals with different metabolites of microalgae that can reduce precursor metal ions into a zerovalent state (Figure 1).
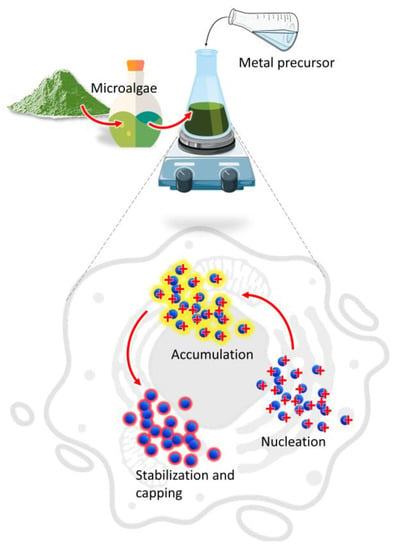
Figure 1. Mechanism of NPs synthesis by microalgae.
The process involves (i) the activation phase, when the metal ion is reduced, and nucleation occurs, followed by (ii) the growth phase, with an amalgamation of formed unit cells into crystallites which is concluded in (iii) the termination phase, where NPs having different shapes and sizes are thermodynamically stable [13]. Other factors such as temperature, pH, or metal ion concentration could affect the synthesis process; however, the participation of microalgae metabolites is crucial to understand the connection between the synthesis procedure and the properties of the obtained product [14,15]. Moreover, during microalgae cultivation sunlight and carbon dioxide can be acquired from the surroundings while the nutrients could be converted from the wastewater to form biomass which provides the new routes for economic sustainability [16].
2.1.1. Proteins
Proteins are an important component in the structure and metabolism of microalgae. They are an integral part of the cellular membrane and light-harvesting complex as well as they participate as enzymes in numerous catalytic reactions [17,18]. Several species of microalgae are studied due to their high protein content ranging from 42–70% in some cyanobacteria and up to 58% for Chlorella vulgaris dry weight [19,20].
The involvement of proteins in the synthesis of nanoparticles is usually investigated using Fourier Transform Infrared Spectroscopy (FTIR) based techniques. The reduction role of the proteins is demonstrated during the oxidation of the −CHO to −COOH group, while NH2 groups usually play capping functions through residual amino acids such as cysteine, tyrosine, and tryptophan [21]. In the study by Chokshi et al., the spectra between the prepared extract of Acutodesmus dimorphus and prepared Ag NPs were compared, showing the role of amide linkage in the stabilization of Ag NPs by peptides and proteins [22]. The obtained NPs were spherical with 2–20 nm in size. Moreover, the overlapping peaks between the extract and the product suggest their coating properties ensure their stabilization and prevent agglomeration. The surface of NPs might be further modified by sulfonated polysaccharides with proteins that provide a link between nanoparticles and coating molecules [21]. Proteins also possess a strong affinity to bind to metal ions that act as reducing agents [21]. Similar findings have been reported for AgCl NPs from Chlorella vulgaris and Ti NPs from marine microalgae Phaeodactylum tricornutum [23,24]. Although the exact mechanism of the synthesis is unknown, the statistical experimental design approach has been studied by using response surface methodology for future large-scale production.
2.1.2. Carbohydrates
Similar to proteins, carbohydrates display both structural and metabolic properties. Mono- and oligosaccharides can be found attached to proteins or lipids, forming glycoproteins or glycolipids, while polysaccharides are the major structural component of the cell wall [25]. Moreover, glucose and starch-like energy storage products are obtained during photosynthesis as the primary carbon-containing molecules in microalgae [26]. Cyanophytes were reported to accumulate glycogen, while other species form semi-amylopectin [27]. Two glucose polymers, amylopectin, and amylose are starch components of Chlorophyta; however, Rhodophyta synthesizes a carbohydrate polymer known as floridean starch [10,28]. Diatoms produce chrysolaminarin composed of β(1,3) and β(1,6) linked glucose units which can accumulate around 7% of their total carbon content in the optimal conditions and up to 80% under strong nutrient depletions [29,30].
Carbohydrates are rich in reducing groups, such as hydroxy and carboxy, which can bind and reduce metal atoms, thereby acting as reducing agents. Moreover, due to the supramolecular interactions by inter- and intra-molecular hydrogen bonding, they can stabilize formed nanoparticles and prevent further agglomeration, acting as capping agents [16,31]. The potential of secretory carbohydrates from C. vulgaris was tested by the removal of biomass from the culture and used for the synthesis of FeOOH NPs [32]. The synthesis process using carbohydrates was compared with the chemical route with sodium hydroxide acting as a precipitating agent. The carbohydrates were reported to be involved in the nucleation process by chelation of iron ions to prevent monotonic nucleation and limit nuclei size, which is further controlled in the growth phase to inhibit large particle formation. The secretory carbohydrates were described mainly for their reducing properties rather than being capping agents. The obtained NPs were spherical with size range 8–17 nm.
The exopolysaccharides from Botryococcus braunii and Chlorella pyrenoidosa were also tested for the synthesis of Ag NPs [33]. The polysaccharides performed both reducing and capping functions and were bound to the Ag NPs surface through carboxy and hydroxy groups. A similar size range was reported of 5–15 nm. In a study by Jakhu et al., Au NPs synthesized from Chlorella sp. polysaccharides were compared with Au NPs synthesized using citrate to compare their properties [34]. Both products exhibited a controlled size range; however, AuNPs from polysaccharides were stable in the pH range 2–12 while citrate-Au NPs were stable only at basic pH values. The NPs synthesized using polysaccharides were significantly bigger than citrate with sie ranges 30–40 nm and 10–15 nm, respectively. Furthermore, citrate-Au NPs were forming agglomerates in a 30-fold lower concentration of NaCl, further proving the stabilization of the surface of Au NPs by microalgal polysaccharides.
2.1.3. Lipids
Secondary to polysaccharide, lipids function as energy reservoirs as well as structural components of the cell membranes. In microalgae, lipids are mainly composed of (i) neural lipids such as free fatty acids, acylglycerols, and carotenoids, and (ii) polar lipids including phospholipids and galactolipids [35]. The polar lipids fraction can significantly increase during exponential growth; however, during stationary phase when the nutrient availability is limited under stress conditions, they can produce triacylglycerols [36]. The fatty acid content is composed of a mixture of C16 and C18 saturated and unsaturated fatty acids with longer carbon-chains including omega fatty acids. Saturated fats are stored in neutral lipid bodies while unsaturated fats are connected with polar lipids in membranes maintaining membrane fluidity under fluctuating cultivation conditions [37,38]. The overall lipid fraction can represent up to 20–50% of the dry biomass, depending on the microalgal species and cultivation conditions such as nutrient availability, salinity, light intensity, and growth phase [39]. During nutrient depletion, the neutral lipid, and polysaccharide content can increase at the expense of proteins [40]. Lipids receive the greatest attention for extraction followed by the production of biodiesel whereas polyunsaturated fatty acids are used for their nutraceutical value.
In contrast to water, which is commonly used for synthesis, the involvement of lipids requires the usage of different solvents. Kashyap et al. utilized ethanolic extract to synthesize Ag/AgCl NPs from Chlorella sp., Lyngbya putealis, Oocystis sp., and Scenedesmus vacuolatus [41]. During the optimization process, Oocystis sp. did not manage to produce NPs while Chlorella sp. extract resulted in the synthesis of Ag/AgCl NPs of the smallest size. The study highlighted the role of lipids and proteins along with the hydroxy group stretching movements in the Ag/AgCl NPs formation with the size range of 10–20 nm. In a study by Gusain et al., lipids and carbohydrates were extracted separately from Acutodesmus obliquus and used for the synthesis of carbon dots by the microwave thermal method [42]. The products had a size range of 1.2–11 nm. The carbon source did not alter the fluorescence behavior; however, the exact interactions during synthesis were not studied. The optical properties changed with the addition of acetone which demonstrated the potential of using different solvents for the synthesis. The role of lipids is hypothesized mainly as capping agents.
2.2. Intracellular Synthesis
Depending on the location where NPs are formed, the corresponding synthesis can be divided into intracellular or extracellular routes. During the former one, live cultures are exposed to the metal precursor, and charged metal ions are transported by negatively charged sites of the cell wall [43]. The trapped ions undergo reduction and form NPs of various sizes and morphologies inside the cell, which require various steps of purification from biomass.
In a study by Li et al., Chromochloris zofingiensis culture was used to prepare Au NPs [44]. The cells after synthesis were characterized showing a peak characteristic for Au NPs in the UV-vis spectrum, which was also confirmed by transmission and scanning electron microscopy findings. The proposed mechanism involves chelation by negatively charged functional groups in the cell wall such as –COOH, –OH, and –OSO3H followed by diffusion into the cytosol and reduction by electrons generated from photosynthetic electron transport using enzymes. Other species with different cell wall structures were also investigated for the synthesis mechanism. Instead, Euglena gracilis species cell wall possesses a glycoprotein-containing pellicle that allowed metal ions to easily penetrate the cell. On the contrary, the marine microalga Nitzschia laevis is composed of a rigid cell wall containing amorphous hydrated porous silica frustule acting as a barrier to reduce ion diffusion into the cells. In addition, Raman spectroscopy was explored as a tool to identify and quantify biomass components in microalgae.
The effect of Ag/AgCl NPs synthesis on chlorophyll and lipid accumulation was studied on freshwater microalgae Scenedesmus sp., showing a decrease of 20–35% after 120 h [45]. However, the cells treated with 0.5 mM AgNO3 showed a 75.86% increase in palmitic acid due to the stress induced by Ag/AgCl NPs. Thus, the cells were able to synthesize Ag/AgCl NPs and improve the quality of biodiesel production. Intracellular synthesis was also used to obtain CdSe quantum dots from C. pyrenoidosa and S. obliquus [46]. First, selenium ions were introduced to the culture to generate selenium precursors within the photosynthetic electron transport system and after 12 h were combined with cadmium ions to form CdSe quantum dots. The algal cells were damaged during the process probably because of precursors reducing enzymatic activity and cell vitality. The intracellular synthesis process requires proper optimization to ensure a high yield while maintaining a low toxicity profile.
2.3. Extracellular Synthesis
Extracellular synthesis utilizes either the secreted molecules such as polysaccharides or involves processing of the biomass to produce extract which is utilized for synthesis of NPs [47]. This route is considered more convenient as NPs are easily purified from the solution. In addition, it allows for further modification of metabolites participating in the synthesis by varying adopted solvents, concentration, time, or pH [48,49].
The cell-free filtrate from freshwater microalgae S. obliquus culture with different nitrogen sources were used to extracellularly synthesize Ag NPs [50]. The study concentrated on the activity of reductases, nitrogen, and sulfate, on Ag NPs synthesis depending on the composition of the medium. The enzymes are conjugated with electron donors and act as reducing agents. Moreover, the activity influenced not only the yield but also the properties or the obtained Ag NPs especially their size. Consequently, their size inversely correlated antimicrobial activity which demonstrates the importance of metabolites during the synthesis.
The cell-free C. vulgaris culture was investigated for different factors affecting Ag NPs synthesis including time, extract/precursor ratio, temperature, pH, precursor molarity, and incubation conditions [51]. The optimal conditions were maximum incubation time (24 h), silver nitrate/extract ratio (8:2), 37 °C, pH 12, 3 mM silver nitrate, and shaking. The study shows the potential of varying multiple factors during the synthesis of NPs, which might result in products with different properties. In a study by Shalaby et al., algal biomass was processed to obtain an extract which provided metabolites implicated in the synthesis of iron oxide NPs [52]. The synthesis was perfected by varying the ratio of precursor to extract, and the product with the highest absorbance was selected for further application.
3. Biomedical Applications of Microalgal NPs
The synthesis route free from toxic waste, as well as economical and environmentally friendly aspects, show the great potential of NPs synthesized from marine and freshwater microalgae for a variety of applications (Figure 2). The presence of naturally occurring biomolecules improves their biocompatibility in comparison with other synthesis routes, and, thus, can be used for biomedical applications. In addition, the growth parameters and metabolites content can be easily altered to obtain a variation in the morphology of NPs for diverse utilization.
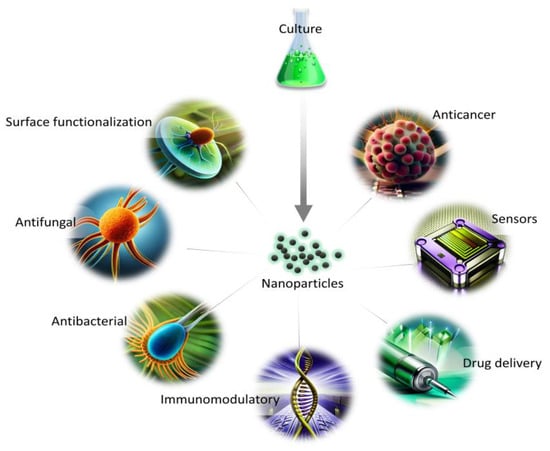
Figure 2. Applications of NPs from microalgae in biomedical fields.
The major drawback connected with the synthesis of NPs using a biological approach is their possible wide size distribution or heterogenous morphology. Thus, the obtained product might be difficult to assess in the context of molecular interactions within tissues or organs. The reason behind the differences might be connected with the complexity of molecules participating in the synthesis which reduce the metal ions with varying efficiency. However, the effect can be minimized by utilizing selected classes of secondary metabolites which can also improve the knowledge of their role in the synthesis.
This entry is adapted from the peer-reviewed paper 10.3390/md21060352
This entry is offline, you can click here to edit this entry!
