Bacterial membrane vesicles (BMVs) are known to be critical communication tools in several pathophysiological processes between bacteria and host cells. Given this situation, BMVs for transporting and delivering exogenous therapeutic cargoes have been inspiring as promising platforms for developing smart drug delivery systems (SDDSs).
1. Introduction
In the classical sense, “drug delivery system” refers to the technologies that carry the active ingredients used for the treatment of any disease into the body. The principal purpose of conventional drug delivery systems is to transport the active molecule in the optimal dose and in a convenient way as well as to facilitate patient compliance and to maintain the stability of the active substance. However, the most notable drawback of conventional systems is that they offer low bioavailability due to the physicochemical properties of the active molecule and they may cause fluctuations in plasma drug level. Drug release from these systems cannot be controlled either [1]. The low water solubility and poor permeability of active molecules as well as rapid metabolization and elimination are associated with their bioavailability, and these features largely limit the pharmacological potential of the active molecules. Therefore, advances in the development of drug delivery systems have always been aimed to increase bioavailability. While we cannot intervene in the physical properties of active molecules, all therapeutic functions can be controlled with the construction of an effective delivery system [2,3].
Since the rise of nanotechnology in the early 2000s and its relationship with drug release technologies, the advantages of “nano-size” over bioavailability have been exploited and many studies have been conducted on nanoscale drug release strategies and their PEGylated derivatives. Today, many commercial examples of “nanocarriers” appear in the world pharmaceutical market. This is simply reasoned by the unique advantages of adaptation of nanotechnology to drug delivery systems, such as the increased release of drugs with low water solubility, facilitated access of the active substance to cells or tissues, efficient passage through epithelial and endothelial barriers, the acquired potential of simultaneous application of multiple substances, and the enabled ability to develop theranostic systems by combining the active substances with imaging agents [
4].
The expectation from a novel drug delivery system involves a specific delivery of the loaded active molecule only to the damaged/diseased tissues and cells where a pharmacological activity is needed. Beyond any doubt, the aim of this approach is strictly to maximize treatment response and minimize side effects. Thus, besides basic concepts such as drug delivery systems, controlled release, and nanotechnology, materials science also comes into play and contributes to emerging smart drug systems strategies. The smart drug delivery phenomenon is mostly based on materials; in many cases polymers with unique properties that sense a change upon external exposure. Later, they initiate a response to the stimuli via different mechanisms depending on the chemical bases of the building-blocks, in other words, they exhibit stimuli-responsive features. This phenomenon can be adapted to the macro, micro, or nanoscales [
5]. Stimuli-responsive drug delivery systems can be created in various organic architectures such as vesicles, polymeric nanoparticles, micelles, dendrimers, or hydrogels as well as inorganic metal oxide frameworks, quantum dots, and mesoporous silica nanoparticles. Basically, the reason why the carrier system is called “smart” is that it reveals the active component it carries as a result of the system’s response to various stimuli. Depending on the source of the stimulus, as endogenous or exogenous, stimuli-responsive materials can be used to effectively deliver the drug of interest [
6,
7,
8,
9].
Bacterial membrane vesicles (BMVs) derived from bacteria have also been recognized as smart drug delivery systems due to their biomimetic properties and versatile functions, as well as the nature of the vesicles, which are important in intercellular communication [
10]. They are distinguished from other nano-sized drug delivery systems as next-generation biomimetic vehicles that can be produced on a large scale at lower costs. Another advantage they provide is that isolated BMVs are amenable to surface modification and allow active targeting via specific ligands, or it is possible to obtain tailor-made BMVs by genetic manipulation on vesicle source strains. Moreover, the most fundamental feature of BMVs that enable them to be used as smart drug delivery systems is their ability to evade the host immune system and maintain the stability of the therapeutic agent [
11].
2. Design and Classification of Smart Drug Delivery Systems
2.1. Design
The main purpose of drug delivery systems is to deliver and target drugs to tissues in a protected manner. The drug given with traditional systems is quickly eliminated from the body. In these cases, regular multiple doses seem to be an alternative, but toxic levels can be observed as a result of overdosing, resulting in poor patient compliance. Smart drug delivery systems (SDDSs) are required to obtain a steady-state concentration in targeted tissues to provide a prolonged effect and eliminate side effects. While designing these systems, there are important parameters such as biomaterial properties, stability, and stimulants. Delivery systems sensitive to stimuli are grouped into two categories: those sensitive to internal and external stimuli [
2].
2.1.1. Internal
Among the internal stimulants, there are pH-responsive, redox-responsive, enzyme-responsive, and ionic microenvironment-responsive systems. In SDDSs designed for stimulation by internal stimuli, drug release occurs with the deterioration of the physicochemical structure of the materials suitable for these stimuli [
12].
pH-responsive. Among the internal stimulants, the pH factor is frequently used [
13]. In pH-responsive biomaterials, deterioration occurs that triggers drug release according to the difference in pH. While in the normal pH range, pH-responsive carriers keep the drug stable and release it with the changing pH in the therapeutic tissues after depots [
2]. Ionizing polymers such as polyacrylic acid and polymethacrylic acid can be used to design pH-responsive carriers. These pH-responsive polymers are categorized as polyacids and poly basics [
14,
15].
Redox-responsive. While the intracellular reduced glutathione (GSH) concentration is between 2 and 10 mM, this ratio is 1/1000 outside the cell. Thus, a redox gradient is formed between the extracellular and the intracellular. The disulfide bond has been recognized as the main binding agent for redox-sensitive systems. While the disulfide bonds in the transporters are stable at low GSH levels in the extracellular environment, they are reduced to thiol groups at high GSH levels inside the cell, and the transporters are disrupted resulting in drug release [
14]. In addition, considering the high accumulation of reactive oxygen species (ROS) in some therapeutic situations, carrier systems have been developed to respond to ROS [
13,
16].
Enzyme-responsive. In certain therapeutic situations, some enzymes are specifically produced at high levels. By using these specific enzymes, SDDSs with high substrate specificity and selectivity have been developed [
13,
17,
18].
Ionic microenvironment-responsive. Ionic microenvironment-responsive carriers are designed by adding acidic and basic functional molecules that affect the ionization power. The presence of highly acidic groups in the carriers creates increased electrostatic repulsion between the negatively charged groups, and at high pH, the physiology of the carrier changes. Thus, drug release is triggered [
12].
2.1.2. External
In the class of external stimulants, some systems are temperature-responsive, light-responsive, electrical field-responsive, magnetic field-responsive, and ultrasound-responsive systems.
Temperature-responsive. Among carriers sensitive to external stimuli, temperature-responsive ones are preferred as a more common strategy. The applicability of naturally occurring and easily generated temperature differences is the main reason for this [
16]. The presence of high temperatures in therapeutic tissues, ranging from 40 to 45 °C, makes the use of temperature-responsive carriers important [
19].
Light-responsive. Ultraviolet light, visible light, and near-infrared light (NIR) are external stimuli used for photosensitive carriers. NIR, with its advantages such as high biocompatibility and in situ polymerization, is an effective stimulant as a photosensitive drug carrier. Different mechanisms have been reported for drug release by systems that respond to NIR: the photo-thermal effect and two-photon activation [
12,
16,
19].
Electrical field-responsive. Heat generation and redox reactions occur as a result of the electrical application in drug delivery systems designed as sensitive to an electric field. In this way, different drug-release pathways are activated by electrical stimulation. Electrical field-responsive polymers such as polypyrrole, polyaniline, and graphene are examples of this system [
2,
12].
Magnetic field-responsive. The magnetic field can penetrate tissues and is frequently used in imaging. Apart from imaging, it has potential as an effective external stimulant for drug delivery systems. Magnetic field-responsive drug delivery systems have magnetic field-induced hyperthermia and magnetic field-directed drug targeting mechanisms. When a magnetic field is applied, heat is generated. Therefore, magnetic nanoparticles are encapsulated in colloidal carriers that trigger drug release in the case of hyperthermia in magnetic field-responsive systems [
2,
12].
Ultrasound-responsive. Ultrasound waves are used as external stimulants in drug release because of their tissue penetration and good spatio-temporal control properties. Ultrasound waves create thermal, mechanical, and radiation forces and these effects trigger drug release in carrier systems [
12].
Dual/Multi-responsive. In addition to drug delivery systems responsive to a single stimulant, combinations of multiple internal or external stimulators can be used to increase efficiency. Among systems responsive to dual stimuli, pH/temperature-responsive systems have been extensively studied. These systems are used in situations that are difficult to target when only temperature-responsive systems are used [
15,
16].
2.2. Classification
There are three main release mechanisms for drug delivery systems: diffusion controlled, osmotically controlled, and chemically controlled. In diffusion-controlled systems, the drug is retained in water-insoluble polymeric membranes or matrices and released by diffusion. Biocompatible membranes with water permeability are used in osmotically controlled systems. In these systems, osmotic pressure is created by the use of NaCl or formulation with an osmogenic effect. Polymers such as cellulose acetate and ethyl cellulose are widely used in osmotically controlled systems. In chemically controlled systems or, in other words, erosion-controlled systems, materials that can degrade in a biological environment are used. There are two types of mechanisms in these systems: polymer-drug dispersion and polymer-drug conjugation. In polymer-drug dispersion, the drug is dispersed into the biodegradable polymer, and drug release occurs with the degradation of the polymer under therapeutic conditions. In the polymer-drug conjugate, the drug is conjugated to the polymer surface by covalent bonds, and drug release occurs by breaking the polymer-drug bonds under therapeutic conditions [
2].
Mesoporous silica systems. Mesoporous silica systems range in size from 50 to 300 nm. Endocytosis allows them to enter the cell without causing cytotoxicity. It has inner and outer surfaces, and these surfaces can be modified to make it functional [
20].
Hydrogels. Hydrogel structures are frequently used in drug delivery. They consist of water-soluble polymers with cross-linked networks. The release of the drug embedded in the hydrogel occurs by the swelling of the polymer in the aqueous medium. Temperature, pH, and the ionic environment are effective in swelling the hydrogel [
18].
Dendrimers. Dendrimers are less common as carrier systems. They have a central core, an inner layer of blocks, and a peripheral region consisting of hyperbranched chains. They can be functionalized with specific ligands to form SDDSs. Its advantages include providing controllable size and molecular weight [
20,
21].
Liposomes. Liposomes are composed of phospholipids arranged in vesicle form. Their size ranges from nanometer sizes to micrometer sizes. Vesicular systems inspired by liposomes can be categorized as niosomes, transfersomes, etosomes, and phytosomes. Niosomes have a non-ionic surface containing low concentrations of phospholipids. Transfersomes are liposomes that have increased flexibility by adding a single chain activator to the surface. If ethanol is used in the preparation of liposomes, they are called etosomes. Finally, if the phospholipids used in the composition are obtained from plants, the structures formed are called phytosomes. The liposome core is suitable for encapsulating hydrophilic drugs, while the peripheral region between the lipid and phospholipid layers encapsulates hydrophobic drugs [
2,
21].
Metal nanoparticles. Metal nanoparticles can be used in the visualization and diagnosis of cellular components as well as in drug delivery. Gold and silver nanoparticles are the most popular. They can be functionalized with bioactive molecules such as antibodies and enzymes. These structures can be included in SDDSs by adding hybrid features [
20].
Polymeric nanoparticles. Polymeric nanoparticles are usually 100–700 nm in size and consist of biodegradable polymer/copolymer structures. Drugs can be encapsulated in these structures, physically absorbed at the surface, or chemically bound to the surface [
20].
Solid-lipid nanoparticles (SLN). Solid lipid nanoparticles have emerged by combining polymeric nanoparticles and liposomes. They can be defined as particles in the nanometer range consisting of biocompatible lipids in solid form at room/body temperature [
2].
Carbon nanotubes (CNT). Carbon nanotubes consist of spherical graphene layers that can be sealed with fullerene. CNTs must be functionalized in order to be used in biological systems; otherwise, they may show cytotoxic effects. Bioactive agents can be conjugated or coated with biodegradable agents to functionalize them [
2,
20].
Polymeric carbon nanoparticles. These are formed by modifying carbon nanotubes with polymers. Polymer wrapping or polymer adsorption can occur based on weak wavelike interactions between carbon nanotubes and polymers, or polymers can be integrated into the carbon nanotube surface by chemical bonds [
20].
Exosomes. Exosomes are vesicles derived from eukaryotic cells that range in size from 30 to 100 nm. It is a biological material and has been frequently studied in recent years due to its ability to enter cells. The advantages of exosomes are their non-cytotoxic, natural targeting abilities and high drug-loading capacity. The disadvantages of exosomes are the difficulty in their large-scale production and purification without losing their biological properties in clinical use [
2,
13,
22].
Bacterial membrane vesicles (BMVs). BMVs are vesicles derived from bacterial cells. BMVs have certain advantages over existing carrier vehicles: they are more stable against leakage and deterioration by keeping the drug more stable in circulation. They can increase the effect of the drug with their immunogenic effects due to the presence of antigen and LPS on their surface. They are highly suitable for surface modification and genetic engineering regulations that will increase targeting efficiency [
23].
3. BMVs as Smart Drug Delivery Systems
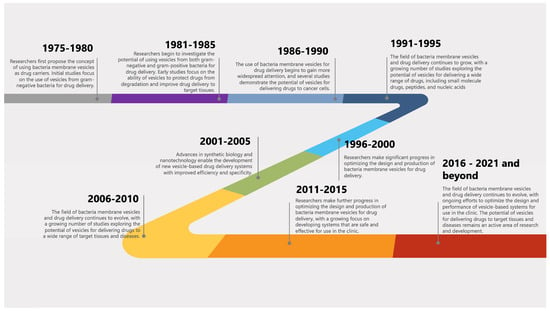
The history and timeline of milestones of BMVs and their applications in drug delivery can be traced back to the late 1970s and early 1980s when the concept of using BMVs as drug carriers was first proposed. A general timeline of key events and milestones in the history of BMVs and their applications in drug delivery is shown in Figure 1. While BMVs show great promise as drug delivery systems, there are still concerns about their stability and toxicity. However, one novel angle to consider is the potential for BMVs to be modified to respond to specific environmental cues within the body, such as pH or temperature changes, to release their contents. This “smart” approach could increase drug efficacy while reducing potential side effects.
Figure 1. A general timeline of key events and milestones in the history of BMVs and their applications in drug delivery.
3.1. Fabrications of BMVs
BMVs are released from both Gram-negative and Gram-positive bacteria. Basically, three models are presented for BMV biogenesis. The first is through the loss of outer membrane and peptidoglycan layer connectivity by the deposition of outer membrane proteins in the periplasmic space. Evidence for this model is the reduction of outer membrane proteins in vesicle-producing bacteria. In the second model, it is assumed that the turgor pressure created by the accumulation of periplasmic proteins and peptidoglycan fragments in the periplasmic space may cause vesicle formation. Finally, vesicle formation is believed to be facilitated by the conformational change of phospholipids on the membrane, leading to a curvature in the membrane [48].
3.2. Purification and Characterization Techniques of BMVs
Serial ultracentrifugation is the most widely utilized method for BMV isolation. This approach involves multiple steps to reach BMVs with the highest purity. First, the bacterial culture is centrifuged at approximately 10,000× g to remove bacterial cell debris. The resulting supernatant is then filtered through a 0.22–0.45 µm filter, and cellular debris is completely removed. Afterward, the cell-free supernatant is ultracentrifuged at 100,000–200,000× g for 1–4 h to obtain BMVs [49]. The disadvantages of this method include long processing times and a loss of integrity in BMVs. In addition, different centrifugal forces and times need to be standardized for this method [50].
Ultrafiltration is another frequently used method in BMV isolation. It is also used to concentrate BMVs following the centrifugation step. This technique provides various advantages such as fast and easy operation, yet it cannot purify the vesicles from other structures with similar size, depending on the pore size of the filter, and therefore may cause contamination. In addition, vesicles may be deformed by the force applied during ultrafiltration [
25].
The precipitation method is a reliable technique for removing macromolecules in solution, and it can also be used for purifying BMVs. To achieve optimal results, precipitation is often combined with ultracentrifugation and ultrafiltration methods. Although the precipitation procedure for BMVs requires multiple steps and additional purification due to the saturation concentration, it is still a cost-effective and efficient option thanks to commercially available precipitation reagents. In fact, a commercial kit specifically designed for BMV isolation is now available [
51].
The ultracentrifugation method is another of the techniques widely used for the purification and isolation of BMVs. The process involves density gradient centrifugation (DGC), where the samples are loaded into a density gradient medium and subjected to centrifugation. This allows for the separation of BMVs based on their density, making it easier to isolate the vesicles through fraction collection. Iodixanol solution is commonly used in this method as it helps preserve the size and shape of the vesicles.
3.3. Properties of BMVs
BMVs are classified based on the gram characteristics of the bacteria from which they are released. Outer membrane vesicles (OMVs) refer to vesicles produced by Gram-negative bacteria, while membrane vesicles (MVs) refer to those produced by Gram-positive bacteria. OMVs range from 30 to 250 nm in diameter and share components with the bacterial outer membrane. OMVs have been found to contain proteins, nucleic acids, and lipids, with various functions attributed to the identified proteins [
60,
61]. On the other hand, MVs range in size from 10 to 500 nm and contain proteins, nucleic acids, and lipids, similar to OMVs [
59,
62].
BMVs have a wide range of functions, which depend on the biomolecules they carry, including adaptation to the environment and pathogenesis. They play a role in transport systems, biofilm formation, antibiotic resistance, phage removal, microbiota protection, gene transfer, pathogenesis, and immune modulation [
59,
62] and also are known to transport various biomolecules, including proteins and virulence factors, between cells. One of the most important advantages over other transport systems is their ability to transport more than one type of biomolecule simultaneously and to transmit these molecules over long distances by protecting them from lytic enzymes [
63]. This property of BMVs makes them particularly effective in the transmission of toxins and virulence factors, which are key factors in bacterial pathogenesis [
64]. The ability of BMVs to carry enzymes such as β-lactamase and drug-binding proteins, and to bind antibiotics outside the cell also contributes to antibiotic resistance in bacteria [
65].
3.4. Cargo Loading or/and Drug Encapsulation Techniques into BMVs
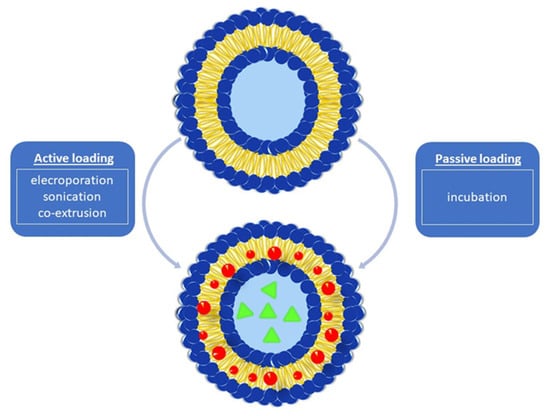
There are two main encapsulation approaches for loading cargo into BMVs. These are energy-dependent (active loading) and energy-independent (passive loading) techniques developed based on the energy requirement principle (Figure 2).
Figure 2. Drug loading approaches into BMVs.
3.4.1. Active Cargo Loading
The active cargo loading principle is an energy-requiring approach and is implemented by electroporation, co-extrusion, and sonication methods. Among these techniques, electroporation is based on the principle of creating pores by breaking the integrity of the membrane in order to create a temporary permeability state in the bacterial cell membrane. For this purpose, short-term electrical voltage pulses are applied to the bacteria cells [
73,
74]. The pores formed in the cell membrane allow the loading of various chemically synthetic drug compounds. By changing the duration and intensity of electrical voltage pulses applied to the cell membrane, low molecular weight synthetic agents, as well as high molecular weight compounds such as nucleotides, can be introduced into the vehicles. After a certain time, the cell membrane returns to its original structure without any damage. This process has also been utilized to entrap active molecules into other vehicles such as exosomes that have a lipid bilayer membrane structure similar to BMVs [
75,
76].
3.4.2. Passive Cargo Loading
Another approach harnessed to load cargo molecules into BMVs is the incubation of drugs with BMVs under appropriate conditions. Kuerban et al. (2020) developed an effective strategy for the treatment of non-small-cell lung cancer with this technique. They aimed to encapsulate doxorubicin hydrochloride (DOX), which is a broad-spectrum antineoplastic agent, into OMVs derived from Gram-negative
Klebsiella pneumoniae. The incubation procedure for loading the active agent in phosphate-buffered saline (PBS) solution into OMVs was performed at 37 °C for a period of 4 h. To remove the unloaded DOX from the incubation medium, an ultrafiltration step was performed through 100 kDa membranes. The DOX:OMV mass ratio of 1:45 was determined as the optimum ratio to obtain the highest encapsulation efficiency. Particle size analysis demonstrated that the mean size of DOX-loaded OMVs was approximately 93 nm. In vitro dissolution studies depicted that DOX was released from OMVs by 30% over 48 h, exhibiting an extended-release profile [
82].
3.5. Drug Release Mechanisms from BMVs
The main release mechanisms by which the release of the active molecule can occur in drug delivery vehicles are diffusion, dissolution, or erosion. It is important to know the mechanism of drug release from BMVs in order to maximize the expected therapeutic response from the carriers and to expand their clinical use. “
Drug release” describes the process by which active molecule solutes can pass the membranes of the BMVs to the dissolution medium or biological fluid. Since the outer membrane structures of BMVs contain lipopolysaccharides, proteins, and lipids, the essential mechanism underlying the release of drugs from BMVs is the diffusion mechanism [
85].
3.6. Strategies Used in BMVs for Targeting
3.6.1. Static Targeting
Passive Targeting
The process of angiogenesis which is necessary for metastasis to occur in tumor development is critical. The development of this process in tumor cells is quite rapid. The endothelial cells of the tumor tissues are not wrapped by pericytes, which ensures the preservation of normal blood vessels, and there is no smooth layer of muscle on the edges of the vessels. Because of this, tumor tissues contain extremely permeable vascular structures also called “
leaky vessels” [
89]. Many solid tumors have excessively permeable vasculature and less lymphatic drainage compared to normal tissues [
90]. Enhanced permeability and retention effect (EPR) which is commonly seen in tumorous areas is characterized by increased capillary permeability in affected tissues with much less return of fluids to the lymphatic circulation and is the main driving power of “
passive targeting” [
91]. While free therapeutic agents can spread non-specifically, a nanocarrier system can accumulate in tumor tissues thanks to leaking vessels with EPR [
92]. Due to the lack of a functional lymphatic drainage system, nanocarriers cannot be efficiently cleared from the bloodstream, so their circulation in the bloodstream is prolonged [
89].
Active Targeting
Since the application site of therapeutics is usually distant from the sites where the therapeutic agent will be affected, the development of effective targeting strategies is critical to therapeutic effectiveness [
95]. In this regard, the research focused on the surface modification of BMVs has opened a new era in SDDSs using biological ligands [
77,
96,
97]. Modified BMVs increase affinity and facilitate the internalization of therapeutic cargo by target cells via receptor-mediated endocytosis and/or disruption of cellular function thanks to an “
active targeting” strategy based on the biological interaction between ligands and the target cell [
98].
3.6.2. Dynamic Targeting
To increase therapeutic efficacy and reduce or avoid adverse effects, drugs must be administered to target sites in a controlled manner. Stimuli-based drug delivery systems have demonstrated significant potential for the effective targeting of active drug moieties in this approach [
109].
Stimuli-Responsive Targeting
To improve drug delivery specificity, efficacy, and biological activities, stimuli-responsive nanocarriers were rationally designed and developed by considering different pathological profiles in normal tissues, intracellular compartments, and tumor microenvironments. Furthermore, it has been reported that stimuli-responsive nanocarriers can overcome multidrug resistance in cancer treatment [
110]. Starting from this, research with BMVs has focused on different internal and external stimuli to trigger drug and/or cargo release.
Dual/Multi-Responsive Targeting
Dual/multi-responsive targeting of BMVs involves the use of targeting moieties that respond to multiple signals, such as pH, temperature, or enzymes, to achieve selective targeting of BMVs. This strategy has the advantage of allowing for multiple triggers to control the release of drugs from BMVs, thereby increasing the level of control and specificity in drug delivery.
One example of a dual/multi-responsive targeting strategy is the combined use of molecules and light-sensitive molecules that will promote the production of ROS as a result of redox reactions as targeting moieties for BMVs. These molecules can be incorporated into the BMVs, and their response to changes in the redox and light of the environment can be used to trigger the release of drugs from BMVs. This allows for a high degree of control over the timing and location of drug release [
114]. A new drug delivery system has been designed that uses αPD-L1-modified OMVs derived from Gram-negative bacteria to deliver the enzyme catalase which breaks down H
2O
2 into O
2, and the photo-sensitive Ce6 molecule together. This nanosystem relieved hypoxia for a long time in vivo by attenuating the hypoxic feature of solid tumors, which inhibited their photodynamic activity.
Inverse Targeting
Inverse targeting of BMVs involves the use of targeting moieties that actively avoid specific regions or conditions, rather than being attracted to them. This strategy is useful for avoiding unwanted toxicity or interactions with non-target cells and could be used to improve the specificity and safety of BMV-based drug delivery.
For example, inverse targeting can be achieved using magnetic nanoparticles that are coated with targeting moieties that bind to vesicles. The magnetic nanoparticles are then functionalized with drugs, and their magnetic properties can be used to direct them away from specific regions or conditions. This allows for targeted delivery of drugs to specific regions of the body while avoiding systemic toxicity.
4. The Role of BMV-Based Smart Drug Delivery Systems in Diagnosis
BMVs are candidates with the potential to be used for bioimaging in the early diagnosis of cancer and evaluation of treatment efficacy due to their ability to encapsulate imaging reporters and target ligands. In this regard, Gujrati and colleagues engineered E. coli cells to overexpress the tyrosine enzyme, resulting in melanin that was contrast-enhancing by optimizing it as a natural light absorber accumulating in the bacteria’s cytosol and packaged in OMVs released from E. coli. Melanin-containing OMVs (OMVMel) capable of generating optoacoustic signals under infrared light induced apoptosis in 4T1 breast cancer cells. In the murine orthotopic breast cancer model, OMVMel exhibited strong necrotic activity by accumulating in tumor tissues with the effect of EPR. These locations have been non-invasively monitored utilizing OMVMel as multispectral optoacoustic tomography (MSOT) probes.
Fusion proteins can be attached to membrane-associated proteins on BMVs through genetic modification and serve many different purposes such as protein transport, fluorescent molecular labeling, tumor therapy, and cell bioimaging [
118]. The OMV-based multifunctional biosensor platform for both antigen targeted, and signal generation developed by Chen et al. (2017) is notable for its simultaneous functionalization of both the interior and exterior of the OMV. To achieve this, the researchers used SlyB lipoprotein as an anchor to direct nanoluciferase (Nluc) interior
E. coli OMVs. The choice of Nluc as a fusion partner was based on its small size of 19 kDa and highly sensitive luminescence signaling. In a novel strategy, the OMVs were operationalized with a target-specific antibody using the ice nucleation protein (INP)-Scaf3 surface scaffolding and the small antibody-binding Z domain on the OMV surface. To test the biosensor’s ability to target cancer cells, the researchers chose the cancer-specific surface marker Mucin-1 (MUC1). They further modified the OMVs by mounting dockerin-labeled green fluorescent protein (GFP) for immunofluorescence imaging on the INP-Scaf3-Z scaffold. When GFP-OMVs were added to HeLa cells stained with anti-MUC1 antibody, bright green fluorescence was detected in cells, demonstrating the OMVs’ ability to detect cancer cells. Overall, this study shows that OMVs can be used for immunofluorescence imaging with target-specific antibodies [
119].
5. The Potential Role of BMV-Based Smart Drug Delivery Systems in Therapy
5.1. Cancer Therapy
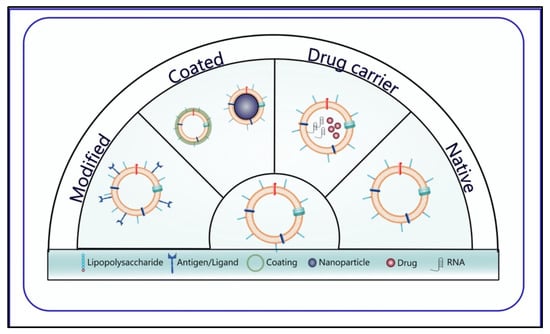
OMVs are utilized in cancer therapy with four different approaches; natural OMVs, cargo and drug-loading OMVs, modified OMVs, and OMVs created with hybrid membrane technology or designed with a coating (Figure 4).
Figure 4. Different OMV approaches used in cancer therapy.
In oncology applications, BMVs offer several advantages. Firstly, their nano sizes ranging from 20 to 200 nm allow for enhanced localization to solid tumors with passive targeting and lymphatic drainage [
126]. Secondly, their EPR properties enable them to accumulate in tumor tissue, triggering local immunity [
127]. Thirdly, BMVs are enriched in pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), found on their surface and originating from the parent bacteria. This natural adjuvant effect activates various toll-like receptor (TLR) signaling pathways, triggering an inflammatory response associated with the activation of the complement system [
48]. Fourthly, BMVs can be genetically engineered through modification of the parent bacteria [
93] with molecular techniques and bioconjugation methods for cell targeting and recognition [
81,
128]. Finally, BMVs cannot reproduce themselves, making them a safer vehicle compared to bacteria [
129,
130].
OMVs appear to be magnificent platforms in bio-applications due to their unique biological properties; even so, more efforts are needed to take them one step further and improve their functionality for practical use. Long-distance transportation and communication are some core functions of OMVs in biological systems [
131,
132]. With the aim of improving their therapeutic functions, research has currently focused on their potential as drug-delivery systems [
87,
133]. OMVs efficiently protect their cargoes against DNase, RNase, protease, and extracellular degradation caused by extreme conditions, such as extreme pH [
134].
Although OMVs play an important role in transporting biomolecules to distant sites, targeting and processing immunomodulatory agents into the tumor microenvironment remains a major challenge. One of the major advantages of using OMVs in cancer therapy is their flexibility to be modified by genetic engineering [
127,
131]. Modifying them is an excellent strategy to improve the expression of specific peptides and the presentation of multiple antigens in targeting cancer cells and forming the tumor-associated immune response.
The functionalization of OMVs with different biomolecules through genetic engineering has several advantages over other methods: (1) There is no need for decoration material because it is created by introducing heterologous DNA into bacteria. (2) Large-scale production of functionalized OMVs can be achieved at a low cost with simple bacterial cultures. As a result, no further purification steps are required after collection. (3) Finally, this method allows the placement of desired biomolecules inside or outside the OMVs according to different application strategies.
Nanoparticles (NPs) have gained significant attention as a promising drug delivery system due to their simple design, low toxicity, and in vivo potency enhancement. However, as exogenous substances, they can be efficiently identified and eliminated by the mononuclear phagocyte system (MPS) in the bloodstream, while their nonspecific distribution can lead to increased toxic effects [
136]. To overcome these limitations, modified NPs in engineered systems have been proposed as a promising strategy.
In recent years, nanoparticle cell membrane coating technology has emerged as a sophisticated and powerful approach to functionalizing NPs [
137]. The approach was initially designed using red blood cell (RBC) membranes to coat polymeric nanoparticles. Subsequently, a broad range of existing cell membrane types such as RBCs, platelets, leukocytes, cancer cells, stem cells, dendritic cells, natural killer cells, and cell membrane-derived structures including exosomes and extracellular vesicles have been comprehensively investigated as nanoparticle coating structures [
138].
5.2. Antimicrobial Therapy
The most common approach to recovering bacterial infections is antibiotic therapy. However, antibiotic treatment cannot be efficient in some cases, e.g., in the case of misuse and self-medication; moreover, it may lead to drug resistance. In this context, the discovery of different antibiotic delivery systems and antibacterial agents is of great importance. The administration of antibiotic therapy is particularly challenging for Gram-negative bacteria due to their double-membrane cell envelope. The ability of BMVs to deliver molecules across the cell envelope of Gram-negative bacteria reveals their antibiotic transport potential [144,145].
The antibacterial effects of OMVs have been documented, with initial evidence provided by Kadurugamuwa and Beveridge in 1996 for lysines in P. aeruginosa-derived vesicles [146]. Subsequent studies have identified several other naturally occurring antibacterial molecules in BMVs from different bacteria. In L. acidophilus, increased amounts of lactacin B in MVs were shown to inhibit the growth of Lactobacillus delbrueckii [147].
Recent studies have shown that, besides their antibacterial properties, BMVs naturally carry antifungal compounds that exhibit antifungal activity. In
Streptomyces albus, genes encoding the synthesis of antifungal compounds, candicidin and antimycin were identified. The production and secretion of these antifungals were observed in the wild-type and mutant strains of
S. albus, and it was discovered that
S. albus MVs carry candicidine [
165].
BMVs can be an intriguing vaccine delivery vehicle for viral antigens because of their self-adjuvant properties and capacity to be adorned with antigens. Finally, studies have evaluated the antiviral abilities of BMVs and their potential as a vaccine platform against viruses. In particular, they have shown promise in protecting against lethal doses of influenza viruses. In a study using BMVs with attenuated lipopolysaccharide content, protection was observed against pandemic H1N1, PR8, and H5N1 virus types, and this antiviral activity was dependent on macrophages, which were induced to produce IFN after BMV administration [
168]. Although the immune response generated by BMVs is temporary, a subsequent study applied BMVs with attenuated lipopolysaccharide content and influenza infections consecutively. This resulted in the induction of virus-specific antibodies in mice, and a second infection at week 4 provided complete protection against viral loading in BMV-treated mice for up to 18 weeks [
169].
6. Safety of BMVs as Smart Drug Delivery Systems
The lack of replicative abilities in BMVs makes them less virulent, yet still potentially harmful due to the presence of virulence factors and toxic components [
148]. A crucial step for BMV applications is the detoxification of toxic components, particularly LPS found on the surface of BMVs [
174]. The LPS consists of acyl chains, core oligosaccharides, lipid A moieties, and O-antigen, with the lipid A moiety being the primary contributor to LPS toxicity [
144]. Detergents are often used to remove LPS from BMVs, which reduces their toxicity. Another approach involves editing genes in bacterial cells to reduce toxicity, such as inactivating the msbB gene or modifying operon genes involved in core oligosaccharide and O-antigen synthesis [
93,
125].
7. Obstacles of BMVs for Clinical Use as Smart Drug Delivery Systems
Biosafety. The use of BMVs in clinical practice faces a major obstacle, which is safety. OMVs are typically derived from Gram-negative bacteria and contain endotoxins and virulence factors such as LPS that can elicit immune responses and toxicity. To address this challenge, genetic engineering has been used to develop OMVs with reduced/attenuated endotoxins by deleting the msbA, msbB, lpxM, and lpxL1 genes [55]. LPS-deficient OMVs exhibit lower immunogenicity than those with normal LPS levels [178], but the optimal balance between low toxicity and high immunogenicity remains a challenge.
Strain selection. OMVs obtained from E. coli are widely used in cancer therapy. However, in using BMVs for these purposes, the identification and selection of specific strains can provide a better balance between the safety and immunogenicity of BMVs. Thus, there is a need for methods to control the immunotoxicity of BMVs to increase their biosafety.
Biogenesis. Literature reports suggest that information on MVs is relatively limited compared to OMVs [
48]. Consequently, the mechanisms of BMV biogenesis remain poorly understood. Further research into the underlying biogenesis mechanisms will aid in discovering and characterizing membrane vesicles from other bacterial species. This will also facilitate the development of improved bacterial-based nanoplatforms for biomedical applications through more in vivo and in vitro studies.
Standardized Production. Obtaining BMVs involves many methods, and there is no standardized analytical protocol. Different production and isolation techniques result in a large heterogeneity of the obtained BMV populations due to the characteristics of BMVs, such as their size and components. This makes it difficult to achieve reproducibility and consistency of results in clinical applications. The interaction of heterogeneous BMVs with target cells and their intracellular fate is unpredictable. Therefore, the development of methods like proteomics and lipidomics to produce homogeneous BMV structures and analyze their composition is critical for their use in clinical applications [
135]. These methods will pave the way for the successful use of BMVs for clinical purposes.
Scalability. The collection and purification of BMVs using the ultracentrifugation method require advanced equipment and qualified personnel, making it time-consuming and costly [
131]. Furthermore, commercial kits available for BMV purification suffer from low yield and purity issues, thereby causing high prices [
179]. Thus, there is an urgent need to develop new and scalable BMV separation/purification methods that can continuously separate BMVs from the culture medium with high yield and purity. Although bacteria can be easily produced in large quantities using large fermentation vessels, the amount of BMVs released from bacteria is not sufficient for their mass production to be cost-effective. Therefore, there is a requirement for high-efficiency production of BMVs to be scalable for clinical applications. To achieve this, different cultural conditions and systems must be explored to optimize BMV production and lay the foundation for BMV industrialization.
Drug-loading. One of the challenges of loading therapeutics into BMVs is the bilayer lipid membrane, which makes it difficult despite various drug-loading strategies. This low loading rate has hindered the clinical application of BMVs. Although Gao et al. performed drug loading with a pH gradient to overcome this situation, they were only able to increase drug loading to 12% [
180]. As a result, new approaches and methods should be developed to increase drug loading to BMVs.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15041052