Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Silanes, as organic-inorganic hybrid modifiers of hyperbranched polymer (HBP), are of great interest as they resulted in a tremendous improvement in HBP properties like increasing thermal, mechanical and electrical properties compared to that of organic-only moieties. The basic components of silanes are monomeric silicon (Si) compounds with four substituent groups attached to the Si atom, which can be of any combination of reactive or non-reactive inorganic or organic groups, which are the fundamental building blocks of silanes.
- silanes
- Hyperbranched polymers
- hybrid modifiers
- coupling agents
1. Introduction
In the Periodic Table, Si belongs to major group 14 along with other elements such as Carbon, Germanium, Tin, Lead and Flerovium, and it has four valence electrons. Si differs chemically from the other elements of Group 14 in terms of structure, reactivity, and, therefore, physical and chemical properties due to its vacant 3D orbitals. Si is classified as a metalloid, as some of its properties are similar to both metals and nonmetals. In the Earth, Si can be found as silica, a variety of silicates and aluminous silicates. With other carbon atoms, carbon can form indefinitely long chains as (-C-C-)n-. On the other hand, Si cannot form long chains but can bond to other Si atoms to form shorter chain lengths, which are unstable in nature [1][2].
This is because the bond energy of the C-C bond is 356 kJ/mol, which is substantially greater than the bond energy of the Si-Si bond (226 kJ/mol). Yet, Si can create arbitrarily long chains to form a siloxane linkage (-O-Si-O-) when combined with oxygen atoms due to the extremely high Si-O bond energy (286 kJ/mol). Si compounds are more highly reactive than carbon compounds as a result of the vacant 3d orbital.
Silanes are compounds made of monomeric Si. An organosilane is a silane with at least one Si-C link (Si-CH3). Through a sequence of reactions, organofunctional silanes are created from SiO2 (silica), the most prominent mineral on our planet Earth. During the reduction of silica to Si, trichlorosilane (HSiCl3) is produced through a reaction between Si and HCl. To create the functional silanes, trichlorosilane first combines with an alkene and then undergoes alcoholysis or a reaction with alcohol.
2. Chemistry of Silanes
Trialkoxysilane, also termed a silane coupling agent, has three alkoxy functional groups at the extremities of its molecular backbone that bind an inorganic substrate to an unpolymerized resin matrix (surface). “R” is an organofunctional group such as amino, vinyl, aryl, methacrylate, acrylate, isocyanato, or sulfo, which furnishes the organic affinity that enables silane groups to produce interpenetrating networks (IPNs). R-(CH2)n-Si-(OX)3 is the general formula of these bi-functional, organofunctional silanes, where “R” is an organofunctional group [3].
Organofunctional silanes are molecules with two distinctly reactive groups linked to the Si atom. This allows them to react and couple to an inorganic surface (such as ceramics and oxide coatings on metals) or to organic resins via a covalent bond. Organofunctional trialkoxysilanes often have the following molecular structure:
R′(CH2)nSi(OX)3, where n = 0, 1, 2, 3 ….
The above Si molecule contains two important varieties of reactive groups: (1) an organofunctional group or organic group (R′) like epoxy, amino, methacryloxy or sulfide, and (2) a hydrolyzable or leaving alkoxy group (OX) like methoxy (-OCH3), ethoxy (-OC2H5), and acetoxy (-OCOCH3). These functional and leaving groups are responsible for the organic compatibility that enables the silane to form IPNs in polymers. -(CH2)- acts as a linker (spacer) group that sits between the organofunctional groups R’ and the Si atom, and the hydrolyzable alkoxyl group is denoted as OX (methoxy, ethoxy).
Before they may connect to the inorganic substrate, silanes are activated by an acid (acetic acid) or undergo hydrolysis to generate silanol groups (SiOH). Two types of moieties are bonded to the Si atom in an organofunctional silane. Alkyl silanes and aryl silanes are used to enhance gloss, concealing ability, mixing time and other attributes linked to their enhanced dispersion. They are additionally used to create hydrophobic surfaces for applications such as water repellents. The “X” stands for alkoxy molecules, most frequently methoxy or ethoxy, which interact with different hydroxyl groups to release methanol or ethanol. To increase coating integrity and adherence, these organofunctional groups (Table 1) offer the linkage with substrates, pigments, matrixes or fillers. The hydroxy functional polymers can also react with the methoxy groups [4][5][6][7][8][9][10]. These organofunctional trialk4oxysilanes undergo two important reactions such as condensation and hydrolysis [6].
Table 1. Examples of organofunctional groups with reactive functional groups.
| S. No | Name of the Silane | Structure | Organofunctional Group Present |
|---|---|---|---|
| 1. | Trichloromethoxy silane | 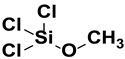 |
Methoxy group |
| 2. | Trimethoxyphenyl silane | 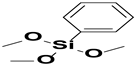 |
Phenyl group |
| 3. | ϒ-mercaptopropyl trimethoxy silane | 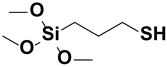 |
Sulfo group |
| 4. | ϒ-aminopropyl trimethoxy silane | 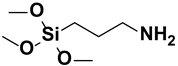 |
Amino group |
| 5. | ϒ- glycidoxypropyl-trimethoxysilane | 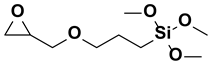 |
Epoxy group |
| 6. | Trichlorovinyl silane | 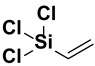 |
Vinyl group |
| 7. | Triethoxyvinyl silane | 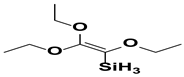 |
Vinyl group |
Hydrolysis: hydrolyzable groups (-OCH3 or –OC2H5) undergo hydrolysis in the presence of water to form silanols (Si-OH).
Condensation: silanols (Si-OH) are condensed together to form a siloxane structure (Si-O-Si) [6].
Meanwhile, the bonding of organofunctional silane molecules to polymers has been studied. Two mechanisms are thought to be responsible for silane’s effective adhesion to polymers: (1) chemical interactions between the reactive groups in the polymers and the organofunctional groups and (2) emergence of IPNs at the silane/polymer interface.
In general, organofunctional silanes act like a “bridge” to encourage adherence between polymers and inorganic substrates (such as glass or oxide coatings on metals). Functional silanes react with polymers to produce chemical bonds and IPNs for good silane/polymer adhesion. In addition, they react with inorganic surfaces to form metallo-siloxane covalent bonds for high adhesion between silanes and inorganic substrates. By reducing water infiltration and bond displacements at the fiber/resin interface, some organofunctional trialkoxysilanes were observed to greatly increase the mechanical strength of composite materials. Organofunctional silanes are now widely used in coatings/paints, adhesives and sealants [8][9].
Before bonding to the substrate, these functional silanes must first be activated by hydrolysis to form silanols. The initial step in the hydrolysis of silanes to silanols (SiOH) is the protonation of alkoxy groups present in silanes. The central Si atom is then the site of a bimolecular nucleophilic substitution (SN2) process. First, a water molecule (a nucleophile) attacks the core Si atom (an electrophile) from the back, resulting in a penta-coordinate trigonal bi-pyramidal transition state. The nucleophile and Si core then form a new bond, and the leaving group (alcohol) and Si center experience a bond cleavage.
3. Classification of Silanes
Silanes are basically classified as functional and non-functional silanes based on the presence of active functional groups. As already detailed, organofunctional silanes will have functional groups such as amino, mercapto and vinyl groups, along with three alkoxy groups. Two separate reactive functional groups are present in organofunctional silanes, which can react and pair with a variety of inorganic and organic compounds. Organofunctional silanes thus serve as adhesion promoters to increase the union of disparate elements. The surface hydroxyl groups of inorganic substrates are reacted with the hydrolyzable functional groups. Organofunctional silanes can react with different functional groups of various compounds [10]. Types of organofunctional silanes and their applications are listed in Table 2.
Table 2. Types of silane and their applications.
| Type of Silane | Applications |
|---|---|
| Alkoxy/chloro silanes | Blocking agent, surface modification and coatings, coupling agent |
| Amino silanes | Coupling agent, adhesion promoter, glass fiber reinforcement, cross-linker, pigment dispersion |
| Phenyl silanes | Coupling agent, industrial coatings, surfactants, hybrid materials |
| Mercapto silanes | Fillers, composites, coupling agents, adhesion promoters |
| Vinyl silanes | Coupling agent, adhesion promoters, crosslinkers |
On the other hand, non-functional silanes will contain only reactive alkoxy (-OR) functional groups. These groups are hydrolyzed to silanol groups and react with the surface hydroxyl groups of inorganic substrates. A bis-functional silane is also known as a crosslinking or dipodal silane and has two Si atoms with three hydrolyzable alkoxy groups on each [11].
4. Factors Affecting Silane Hydrolysis
The molecular structure of the silane, its concentration, pH, temperature, humidity and solvent system are some of the important factors that affect the rate of silane hydrolysis. With increasing alkoxy group size, the hydrolysis rate decreases in the order of pentoxy > butoxy > propoxy > ethoxy > methoxy. pH has a significant impact on silane hydrolysis. The rate of silane hydrolysis is rapid in acidic and alkaline media, although it is at its lowest for alkoxysilanes at neutral pH [12].
The hydrolysis reaction rate rises as the temperature rises, following the Arrhenius law. The type of co-solvent in the solvent combination also affects the hydrolysis rate. The hydrophilicity of the solvent affects the rate of hydrolysis. The hydrolysis rate of α-silanes and γ-silanes reduces when the hydrophilicity of methanol, ethanol and propan-1-ol increases. The molecular structure of the terminal silyl group is the cause of the slow crosslinking kinetics of conventional silane-terminated polymers.
The moisture-induced crosslinking reaction occurs much more slowly with γ-alkoxysilanes than it does with the extremely reactive α-alkoxysilanes. The electron donor is joined to the Si atom in α-silanes by a methylene group. The alkoxy groups are activated in this arrangement, greatly accelerating the crosslinking reaction. Organofunctional alkoxysilanes contain at least one alkoxy group as one of the four groups linked to the Si atom (–OR). Bi-functional (two alkoxy group) and tri-functional (three alkoxy group) silanes are distinguished based on the number of alkoxy groups. Alkoxy groups have the capacity to hydrolyze. A siloxane network is created during the reaction with water. In addition to the alkoxy groups, there is a functional organic group on the Si atom (R) through which a silane can also bind to an organic molecule. The length of the hydrocarbon chain (spacer) in the reactive organic group is a crucial structural component of organofunctional alkoxysilanes. The length of the hydrocarbon chain has a major influence on how firmly the alkoxy groups are bound to the Si atom and, thus, on the speed of crosslinking in the presence of moisture [10].
This entry is adapted from the peer-reviewed paper 10.3390/polym15112517
References
- Semenov, V. V Alkanes and Silanes: Similarities and Differences. Her. Russ. Acad. Sci. 2016, 86, 466–472.
- Uneyama, K. Functionalized Fluoroalkyl and Alkenyl Silanes: Preparations, Reactions, and Synthetic Applications. J. Fluor. Chem. 2008, 129, 550–576.
- Goyal, S. Silanes: Chemistry and Applications. J. Indian Prosthodont. Soc. 2006, 6, 14–18.
- Shokoohi, S.; Arefazar, A.; Khosrokhavar, R. Silane Coupling Agents in Polymer-Based Reinforced Composites: A Review. J. Reinf. Plast. Compos. 2008, 27, 473–485.
- Kateklum, R.; Gauthier-Manuel, B.; Pieralli, C.; Mankhetkorn, S.; Wacogne, B. Improving the Sensitivity of Amino-Silanized Sensors Using Self-Structured Silane Layers: Application to Fluorescence PH Measurement. Sens. Actuators B Chem. 2017, 248, 605–612.
- Brochier Salon, M.-C.; Abdelmouleh, M.; Boufi, S.; Belgacem, M.N.; Gandini, A. Silane Adsorption onto Cellulose Fibers: Hydrolysis and Condensation Reactions. J. Colloid Interface Sci. 2005, 289, 249–261.
- Issa, A.A.; Luyt, A.S. Kinetics of Alkoxysilanes and Organoalkoxysilanes Polymerization: A Review. Polymers 2019, 11, 537.
- Máková, V.; Holubová, B.; Krabicová, I.; Kulhánková, J.; Řezanka, M. Hybrid Organosilane Fibrous Materials and Their Contribution to Modern Science. Polymers 2021, 228, 123862.
- Zhu, D.; Hu, N.; Schaefer, D.W. Water-Based Sol–Gel Coatings for Military Coating Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128142011.
- Gadhave, R.V.; Gadhave, C.R.; Dhawale, P.V. Silane Terminated Prepolymers: An Alternative to Silicones and Polyurethanes. Open J. Polym. Chem. 2021, 11, 31–54.
- Osterholtz, F.D.; Pohl, E.R. Kinetics of the Hydrolysis and Condensation of Organofunctional Alkoxysilanes: A Review. J. Adhes. Sci. Technol. 1992, 6, 127–149.
- Gadhave, R.V.; Sheety, P.; Mahanwar, P.A.; Gadekar, P.T.; Desai, B.J. Silane Modification of Starch-Based Wood Adhesive: Review. Open J. Polym. Chem. 2019, 09, 53–62.
This entry is offline, you can click here to edit this entry!
