Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
A fiber optic evanescent wave (FOEW) sensor is an attractive type of portable device that has the advantages of high sensitivity, low cost, good reusability, and long-term stability. By utilizing functional nucleic acids (FNAs) such as aptamers, DNAzymes, and rational designed nucleic acid probes as specific recognition ligands, the FOEW sensor has been demonstrated to be a general sensing platform for the onsite and continuous detection of various targets ranging from small molecules and heavy metal ions to proteins, nucleic acids, and pathogens.
- functional nucleic acid
- evanescent wave biosensors
- optical fiber
- aptamers
- DNAzymes
1. Introduction
Onsite continuous detection techniques are highly desired for real-time monitoring of pollutants in environmental waters [1] and food production processes [2][3]. They also are of central importance for the development of wearable medical devices to routinely measure biomarkers [4][5]. These technical advances are dramatically changing traditional lab-based strategies into more and more convenient, automatic, and labor-free means. To realize onsite continuous detection for daily use, a technology should be sensitive, specific, affordable, automated, rapid, and reusable, criteria referred to as “2-SAR”. Numerous biosensors have been reported in recent years with the overall purpose to achieve high sensitivity or rapidity. Quite often, expensive and complicated signal amplification strategies, most commonly enzymatic reactions, are used to enhance the detection sensitivity. In addition, tedious sample processing steps prior to detection are required to avoid matrix interference. These offline processes greatly lengthen the assay time and complicate the operation, preventing their application for continuous detection, where the timely monitoring of the concentration of the target is required. Ideally, the detection should be reagent-free, where no reagents need to be added for signal amplification or sensor regeneration.
Of the existing sensing platforms, only a few meet the “2-SAR” criteria and are commercially available [6]. The most outstanding example is the wearable electrochemical sensor for the real-time detection of blood sugar levels [7][8]. The continuous detection is based on the electrochemical signals generated by the in situ oxidation reactions of glucose catalyzed by the enzymatic electrode. The technique is elegant, but only suitable for targets that can undergo specific redox reactions. Most pollutants or biomarkers do not have redox activity under mild conditions and the specific enzymes are also not available. Moreover, the sensitivity of this technique is in the low millimole per liter (mM) concentration range, which does not meet the sensitivity requirements for most pollutants and biomarkers (typically in the picomole to nanomole per liter concentration range). Electrochemical devices are also commercially available for the onsite detection of heavy metal ions [9]. They are based on the redox reaction of metal ions, rendering high sensitivity and specificity. The limitation is the interference from the sample matrix resulting from the nonspecific absorption of various components on the electrode, which interferes with the current. Therefore, offline sample pretreatment is commonly required to ensure the reusability of the electrodes, leading to the long assay time and the high cost. Fluorescent evanescent wave sensors are another type of portable optical sensors that are suitable for continuous detection [6][10][11][12][13]. They are based on the evanescent wave generated on the surface of waveguide materials (chip or fiber) upon laser incidence from the light-dense medium to the light-sparse medium. Planar waveguide sensors are capable of the simultaneous detection of multiplex targets but require a much more sophisticated and expensive optical system than fiber optical evanescent wave (FOEW) sensors [14]. Fluorescent FOEW sensors are, therefore, more suitable for continuous detection merely from an affordability point of view. In fact, fluorescent FOEW sensors have attracted more attention than planar waveguide sensors in recent years. Over the past half-century, tremendous advances have been made in FOEW sensors, from the optical system, fluid system, to the modification chemistry of optical fiber and sensing mechanisms [6][10][11][12][13].
Functional nucleic acids (FNAs) include aptamers, DNAzymes, and rationally designed nucleic acids, which all have the ability to specifically recognize targets and are widely used in various types of biosensors [15]. Due to rapid advances in FNAs in the last thirty years, the application scope of FOEW sensors has been rapidly expanding from common water quality parameters including pH, temperature, oxygen, ions, refractive index, and (dissolved) gases and vapors to almost any type of target. FNA-FOEW sensors have been reported for the detection of all types of targets including metal ions, small molecules, proteins, nucleic acids, bacteria, and viruses with the promise of more affordable, durable, and flexible detection compared to antibody-based FOEW sensors. The sensitivity and dynamic range of all FNA-based sensors are strongly affected by the probe density on the sensor surface [16]. FNA-based electrochemical sensors are typically orders of magnitude more sensitive than FOEW, but they are difficult to regenerate. In contrast, FNA-FOEW sensors can be regenerated, enabling repeated measurements using the same sensor and, therefore, a higher accuracy.
2. Functional Nucleic Acids (FNAs)
Nucleic acids were initially defined as a hereditary substance that carries genetic information. Later, research clearly evidenced that some nucleic acids, called FNAs, also play many other important biological functions in vivo or in vitro [15][17][18][19][20]. FNAs with catalytic activity or specific molecular recognition capability have attracted tremendous attention in many fields. They are divided into two categories: natural and artificial FNAs. Natural FNAs are found in vivo and include ribozymes and riboswitches. Artificial FNAs are obtained by in vitro screening or engineering, and include rationally designed nucleic acid probes, aptamers, DNAzymes, and aptazymes (Figure 1). Artificial FNAs are attractive synthetic probes because of their low cost, ease of synthesis and modification, high stability, and biocompatibility. They have been extensively used for sensing detection [21][22], drug delivery [23] and disease therapy [17], molecular imaging [24], and self-assembly of nanomaterials [25][26]. Among these applications, their applications in fluorescent, colorimetric, and electrochemical sensors are dominant and have shown the greatest potentials for practical uses.
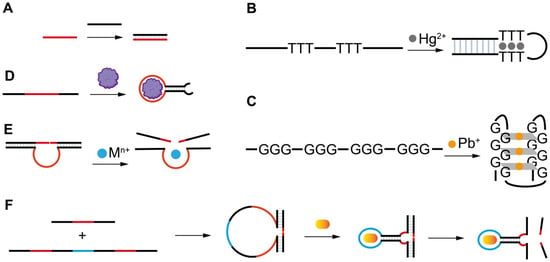
Figure 1. The artificial FNAs most popularly used in biosensors including FNA-FOEW sensors. (A) Complementary DNA probe (cDNA); (B) rationally designed thymine (T)-rich probe for highly specific Hg2+ detection; (C) rationally designed guanine (G)-rich probe for highly specific Pb2+ detection; (D) aptamers; (E) DNAzymes specific to various metal ions; (F) aptazymes for nonmetal ion targets.
The different types of FNAs enable the specific recognition of different types of targets. The first groups of FNAs are short complementary DNA probes (cDNAs) (Figure 1A), typically 10–25 mer in length, which are rationally designed to be completely or partially complementary to the target DNA or RNA sequences. The hybridization reactions between cDNAs and target sequences cause the formation of duplex structures in a solution or on the sensor surface, allowing the specific detection of target sequences by various signal transformation means. The hybridization reaction has also been extended for the indirect detection of other types of targets beyond nucleic acids by incorporating the following FNAs. Another type of rationally designed FNA sequence is metal-ion-specific DNA probes. The most applied ones are thymine (T)-rich probes highly specific to Hg2+ [27][28][29] (Figure 1B) and guanine (G)-rich probes highly specific to Pb2+ [30][31][32][33] (Figure 1C). The two types of probes respectively undergo a large conformation change from random coil to duplex or G-quadruplex structures upon binding to Hg2+ or Pb2+. Rationally designed probes have also been reported for other metal ions, such as Ag+ [34] and K+ [35][36], but are much less applied because they are not the most common pollutants of concern in the environment or food.
Both aptamers and DNAzymes are in vitro isolated nucleic acids from a nucleic acid library by SELEX (Systematic Evolution of Ligands by Exponential Enrichment) technology [22][37][38]. Aptamers are specific binding ligands without catalytic activity (Figure 1D). Since the invention of SELEX, more than one thousand aptamers have been isolated, targeting all types of targets from metal ions, small molecules, and proteins to complex targets such as bacteria and viruses [39][40][41][42]. The specific binding event can be signally transformed in diverse sensing platforms. DNAzymes are FNAs with diverse enzymatic activities. DNAzymes with cleavage activity catalyzed by a wide spectrum of metal ions as cofactors have been reported in recent years [22][43] (Figure 1E). Among them, the most popularly used are those specific to Pb2+. The DNAzyme forms two duplex segments with its substrate, leaving the central loop region as the metal ion recognition pocket. In the presence of the right metal ion, the cleavage activity is activated due to the binding between the metal ion and the loop, and the phosphodiester bond next to the RNA nucleotide in the substrate sequence is cleaved. The catalytic cleavage of the substrate sequence lays the basis for various sensing mechanisms. Due to their catalytic property, one metal ion can trigger multiple cycles of cleavage, leading to high sensitivity and negating the use of protein enzymes for signal amplification.
Aptazymes are a type of FNA with both catalytic activity and specific binding capability, in which the binding pocket in DNAzymes for metal ions is designed through engineering to include aptamer sequences [44] (Figure 1F). Even though the design is elegant, tedious optimization is required and their extended applications are limited. So far, no aptazymes have been used in FOEW sensors.
In FOEW sensors, the above-described FNAs are either functionalized on the fiber surface or added into the test sample. The fluorescent group is typically modified on the FNAs or their complementary sequences for signal transduction of the specific molecular recognition event that occur on the fiber surface.
3. The Major Components of Fluorescent FOEW Sensors and Optical Mechanisms for Real-Time Fluorescence Detection
A typical fluorescent FOEW sensor consists of an optical system (laser, optical fiber coupler, filter, photodiode, and signal amplifier), a mechanic fluidic system (peristaltic pump, tubes, reaction chamber), and a data analysis system (software and computer) (Figure 2A) [6]. The optical fiber is installed inside the reaction chamber. The inlet and outlet tubes are connected to the chamber for automatic sample injection and waste elution with controlled flow rate and time [45]. For the purpose of portability, the FOEW sensors have been miniaturized and several portable FOEW sensors have been commercialized for in situ applications [10]. Continuous onsite measurements are realized by the regeneration of the sensor surface by simply rinsing with sodium dodecyl sulfate solution (0.5% SDS, pH 1.9) after each measurement [46]. Very recently, the miniaturized all-fiber-optical system and microfluidic system have been integrated with smartphones for onsite real-time quantitative detection of bisphenol A and norfloxacin in 15 min with high sensitivity and reusability, and automated interpretation of reporting results [47].
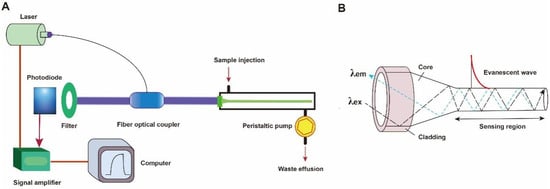
Figure 2. (A) The components of a typical fluorescent FOEW sensor; (B) the structure of the tapered fiber and the evanescent wave generated vertical to the fiber surface.
The optical fiber is the core element of an FOEW sensor for real-time fluorescence sensing. It usually consists of the inside core and the outside cladding. The refractive index (RI) of the fiber core is higher than that of the fiber cladding. Light travels through the fiber via total internal reflection. The material of the core is usually transparent glass (SiO2) or plastic with excellent laser transport capability and ease of surface modification. With the purpose of improving sensitivity, optical fibers with diverse shapes have been reported. They can be roughly divided into two categories, fiber gratings (FGs) and structured optical fibers (SOFs) [11]. FGs are optical fibers that have periodic gratings, which change the refractive index (RI) of the core. Common SOFs include D-shaped, U-shaped, tapered, and biconical fibers (Figure 3). The SOFs, especially the tapered fiber, are much easier to fabricate than FGs. Optical fibers for the preparation of tapered fibers are commercially available at quite a low price (approximately USD 1–2 per probe). For this reason, tapered fibers are the most widely used fibers in FOEW sensors.
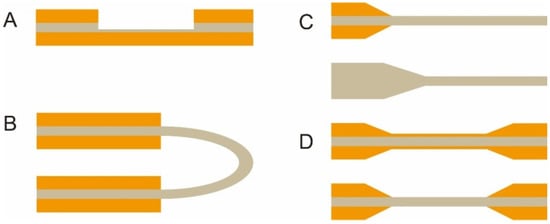
Figure 3. The most common optical fiber structures. (A) D-shaped fibers; (B) U-shaped fibers; (C) tapered fibers; (D) biconical fibers. The gray part is the core, and the orange part is the cladding. The section without the cladding is the sensing region, where the surface modification is typically performed prior to the sensing.
Taking the tapered fiber as an example, the generation of the optical phenomenon inside and on the surface of the fiber was illustrated (Figure 2B). For other shapes of optical fibers, an excellent review has introduced them in detail [11]. When the laser incidence from the light-dense medium (such as SiO2) to the light-sparse medium (such as an aqueous sample) and the angle of incidence is greater than the critical angle, the refracted light disappears, and total reflection occurs. The total reflection of the incident laser inside the fiber leads to the formation of the evanescent wave field propagated vertical to the surface of the fiber. The distance is called the depth of penetration (dp) when the intensity of the evanescent wave decays to 1/e of the original light wave intensity [48]. The dp is a function of several parameters as shown in the following equation and is typically in the 100–200 nm range.
where λ represents the wavelength of the incident light; n1 represents the refractive index of the fiber core medium; n2 represents the refractive index of the surrounding solution medium; and θ is the angle of incidence of the fiber at the interface. A large dp is the key to achieve high sensitivity for FOEW sensors. The tapering, launch angle, and taper length of the fiber strongly affect the sensitivity and can be easily optimized.
The evanescent wave excites the fluorescence emission of fluorophores inside the evanescent wave field. The intensity of the emission fluorescence is then real-time measured by the photodiode detector after the filter. It is worth pointing out that FOEW sensors tend to show a higher signal-to-noise (or lower background interference) ratio compared to the fluorescence measurements conducted in solution because only the fluorophores within the evanescent wave field can be effectively excited [6].
In order to realize the simultaneous detection of multiple targets, Long et al. designed and manufactured a compact dual-color FOEW sensor installed with two lasers for the simultaneous excitation of two fluorophores at distinct wavelengths. The simultaneous detections of aflatoxin M1 (AFM1) and ochratoxin A (OTA) [49], Escherichia coli (E. coli) O157:H7 and Salmonella typhimurium [50], or acetamiprid and fipronil [51] were respectively achieved. The group further simplified the optical structure, where a single-multi mode fiber optic coupler was employed to replace the sophisticated confocal optical system for the transmission of two excitation lights and dual-color fluorescence [52][53]. A photodiode detector was used instead of a photomultiplier for the simultaneous detection of dual-color fluorescence.
Besides the solid fibers described above, a hollow-core fiber has also been used in fluorescent FNA-FOEW sensors [54]. Wang et al. recently demonstrated that hollow-core microstructured antiresonant fibers (HARFs) can stringently confine light in the fiber core, ensuring a high signal and sensitivity. The hollow-hole fiber or capillary serving as a waveguide was first invented in 2000 by Liger et al., and has been commercialized.
This entry is adapted from the peer-reviewed paper 10.3390/bios13040425
References
- Hui, Y.; Huang, Z.; Alahi, M.E.E.; Nag, A.; Feng, S.; Mukhopadhyay, S.C. Recent advancements in electrochemical biosensors for monitoring the water quality. Biosensors 2022, 12, 551.
- Faraji Rad, Z. Microneedle technologies for food and crop health: Recent advances and future perspectives. Adv. Eng. Mater. 2022, 25, 2201194.
- Medina, S.; Perestrelo, R.; Silva, P.; Pereira, J.A.M.; Câmara, J.S. Current trends and recent advances on food authenticity technologies and chemometric approaches. Trends Food Sci. Technol. 2019, 85, 163–176.
- Chung, M.; Fortunato, G.; Radacsi, N. Wearable flexible sweat sensors for healthcare monitoring: A review. J. R. Soc. Interface 2019, 16, 20190217.
- Teymourian, H.; Parrilla, M.; Sempionatto, J.R.; Montiel, N.F.; Barfidokht, A.; Van Echelpoel, R.; De Wael, K.; Wang, J. Wearable electrochemical sensors for the monitoring and screening of drugs. ACS Sens. 2020, 5, 2679–2700.
- Taitt, C.R.; Anderson, G.P.; Ligler, F.S. Evanescent wave fluorescence biosensors. Biosens. Bioelectron. 2005, 20, 2470–2487.
- Adeel, M.; Rahman, M.M.; Caligiuri, I.; Canzonieri, V.; Rizzolio, F.; Daniele, S. Recent advances of electrochemical and optical enzyme-free glucose sensors operating at physiological conditions. Biosens. Bioelectron. 2020, 165, 112331.
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanovic, G.M. Comprehensive review on wearable sweat-glucose sensors for continuous glucose monitoring. Sensors 2022, 22, 638.
- Ding, Q.; Li, C.; Wang, H.; Xu, C.; Kuang, H. Electrochemical detection of heavy metal ions in water. Chem. Commun. 2021, 57, 7215–7231.
- Taitt, C.R.; Anderson, G.P.; Ligler, F.S. Evanescent wave fluorescence biosensors: Advances of the last decade. Biosens. Bioelectron. 2016, 76, 103–112.
- Jiao, L.; Zhong, N.; Zhao, X.; Ma, S.; Fu, X.; Dong, D. Recent advances in fiber-optic evanescent wave sensors for monitoring organic and inorganic pollutants in water. TrAC Trends Anal. Chem. 2020, 127, 115892.
- Wang, X.D.; Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors (2013–2015). Anal. Chem. 2016, 88, 203–227.
- Wang, X.D.; Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors (2008–2012). Anal. Chem. 2013, 85, 487–508.
- Benito-Pena, E.; Valdes, M.G.; Glahn-Martinez, B.; Moreno-Bondi, M.C. Fluorescence based fiber optic and planar waveguide biosensors. A review. Anal. Chim. Acta 2016, 943, 17–40.
- Liu, J.; Cao, Z.; Lu, Y. Functional nucleic acid sensors. Chem. Rev. 2009, 109, 1948–1998.
- Ravan, H.; Kashanian, S.; Sanadgol, N.; Badoei-Dalfard, A.; Karami, Z. Strategies for optimizing DNA hybridization on surfaces. Anal. Biochem. 2014, 444, 41–46.
- Peng, T.; Deng, Z.; He, J.; Li, Y.; Tan, Y.; Peng, Y.; Wang, X.-Q.; Tan, W. Functional nucleic acids for cancer theranostics. Coord. Chem. Rev. 2020, 403, 213080.
- Alsaafin, A.; McKeague, M. Functional nucleic acids as in vivo metabolite and ion biosensors. Biosens. Bioelectron. 2017, 94, 94–106.
- Liu, R.; McConnell, E.M.; Li, J.; Li, Y. Advances in functional nucleic acid based paper sensors. J. Mater. Chem. B 2020, 8, 3213–3230.
- Li, Y.; Yi, L. Functional Nucleic Acids for Analytical Applications; Springer: Berlin/Heidelberg, Germany, 2009.
- Mok, W.; Li, Y. Recent progress in nucleic acid aptamer-based biosensors and bioassays. Sensors 2008, 8, 7050–7084.
- Zhou, W.; Saran, R.; Liu, J. Metal sensing by DNA. Chem. Rev. 2017, 117, 8272–8325.
- Zhao, H.; Yuan, X.; Yu, J.; Huang, Y.; Shao, C.; Xiao, F.; Lin, L.; Li, Y.; Tian, L. Magnesium-stabilized multifunctional DNA nanoparticles for tumor-targeted and pH-responsive drug delivery. ACS Appl. Mater. Interfaces 2018, 10, 15418–15427.
- Wu, Y.; Yang, Z.; Lu, Y. Photocaged functional nucleic acids for spatiotemporal imaging in biology. Curr. Opin. Biotechnol. 2020, 57, 95–104.
- Lu, Y.; Liu, J. Functional DNA nanotechnology: Emerging applications of DNAzymes and aptamers. Curr. Opin. Biotechnol. 2006, 17, 580–588.
- Zhang, J.; Lan, T.; Lu, Y. Molecular engineering of functional nucleic acid nanomaterials toward in vivo applications. Adv. Healthcare Mater. 2019, 8, 1801158.
- Ono, A.; Togashi, H. Highly selective oligonucleotide-based sensor for mercury (II) in aqueous solutions. Angew. Chem. Int. Ed. Engl. 2004, 43, 4300–4302.
- Du, J.; Liu, M.; Lou, X.; Zhao, T.; Wang, Z.; Xue, Y.; Zhao, J.; Xu, Y. Highly sensitive and selective chip-based fluorescent sensor for mercuric ion: Development and comparison of turn-on and turn-off systems. Anal. Chem. 2012, 84, 8060–8066.
- Lou, X.; Zhao, T.; Liu, R.; Ma, J.; Xiao, Y. Self-assembled DNA monolayer buffered dynamic ranges of mercuric electrochemical sensor. Anal. Chem. 2013, 85, 7574–7580.
- Peng, D.; Li, Y.Q.; Huang, Z.C.; Liang, R.P.; Qiu, J.D.; Liu, J.W. Efficient DNA-catalyzed porphyrin metalation for fluorescent ratiometric Pb2+ detection. Anal. Chem. 2019, 91, 11403–11408.
- Xu, L.; Shen, X.; Hong, S.; Wang, J.; Zhang, Y.; Wang, H.; Zhang, J.; Pei, R. Turn-on and label-free fluorescence detection of lead ions based on target-induced G-quadruplex formation. Chem. Commun. 2015, 51, 8165–8168.
- Li, T.; Dong, S.J.; Wang, E.K. A lead(II)-driven DNA molecular device for turn-on fluorescence detection of lead(II) ion with high selectivity and sensitivity. J. Am. Chem. Soc. 2010, 132, 13156–13157.
- Li, T.; Wang, E.K.; Dong, S.J. Lead(II)-induced allosteric G-quadruplex DNAzyme as a colorimetric and chemiluminescence sensor for highly sensitive and selective Pb2+ detection. Anal. Chem. 2010, 82, 1515–1520.
- Ono, A.; Cao, S.; Togashi, H.; Tashiro, M.; Fujimoto, T.; Machinami, T.; Oda, S.; Miyake, Y.; Okamoto, I.; Tanaka, Y. Specific interactions between silver(I) ions and cytosine-cytosine pairs in DNA duplexes. Chem. Commun. 2008, 39, 4825–4827.
- He, F.; Tang, Y.L.; Wang, S.; Li, Y.L.; Zhu, D.B. Fluorescent amplifying recognition for DNA G-quadruplex folding with a cationic conjugated polymer: A platform for homogeneous potassium detection. J. Am. Chem. Soc. 2005, 127, 12343–12346.
- Zheng, D.; Zou, R.; Lou, X. Label-free fluorescent detection of ions, proteins, and small molecules using structure-switching aptamers, SYBR gold, and exonuclease I. Anal. Chem. 2012, 84, 3554–3560.
- Wang, T.; Chen, C.; Larcher, L.M.; Barrero, R.A.; Veedu, R.N. Three decades of nucleic acid aptamer technologies: Lessons learned, progress and opportunities on aptamer development. Biotechnol. Adv. 2019, 37, 28–50.
- Willner, I.; Shlyahovsky, B.; Zayats, M.; Willner, B. DNAzymes for sensing, nanobiotechnology and logic gate applications. Chem. Soc. Rev. 2008, 37, 1153–1165.
- Yu, H.; Alkhamis, O.; Canoura, J.; Liu, Y.; Xiao, Y. Advances and challenges in small-molecule DNA aptamer isolation, characterization, and sensor development. Angew. Chem. Int. Ed. Engl. 2021, 60, 16800–16823.
- Guo, W.; Zhang, C.; Ma, T.; Liu, X.; Chen, Z.; Li, S.; Deng, Y. Advances in aptamer screening and aptasensors’ detection of heavy metal ions. J. Nanobiotechnol. 2021, 19, 166.
- Majdinasab, M.; Hayat, A.; Marty, J.L. Aptamer-based assays and aptasensors for detection of pathogenic bacteria in food samples. TrAC Trends Anal. Chem. 2018, 107, 60–77.
- Lou, B.; Liu, Y.; Shi, M.; Chen, J.; Li, K.; Tan, Y.; Chen, L.; Wu, Y.; Wang, T.; Liu, X.; et al. Aptamer-based biosensors for virus protein detection. TrAC Trends Anal. Chem. 2022, 157, 116738.
- Lake, R.J.; Yang, Z.; Zhang, J.; Lu, Y. DNAzymes as activity-based sensors for metal Ions: Recent applications, demonstrated advantages, current challenges, and future directions. Acc. Chem. Res. 2019, 52, 3275–3286.
- Liu, J.W.; Lu, Y. Adenosine-dependent assembly of aptazyme-functionalized gold nanoparticles and its application as a colorimetric biosensor. Anal. Chem. 2004, 76, 1627–1632.
- Zhao, J.; Lu, Z.; Wang, S.; Wei, Z.; Zhou, J.; Ren, S.; Lou, X. Nanoscale affinity double layer overcomes the poor antimatrix interference capability of aptamers. Anal. Chem. 2021, 93, 4317–4325.
- Long, F.; Gao, C.; Shi, H.C.; He, M.; Zhu, A.N.; Klibanov, A.M.; Gu, A.Z. Reusable evanescent wave DNA biosensor for rapid, highly sensitive, and selective detection of mercury ions. Biosens. Bioelectron. 2011, 26, 4018–4023.
- Cheng, Y.; Wang, H.; Zhuo, Y.; Song, D.; Li, C.; Zhu, A.; Long, F. Reusable smartphone-facilitated mobile fluorescence biosensor for rapid and sensitive on-site quantitative detection of trace pollutants. Biosens. Bioelectron. 2022, 199, 113863.
- Ahmad, M.; Hench, L.L. Effect of taper geometries and launch angle on evanescent wave penetration depth in optical fibers. Biosens. Bioelectron. 2005, 20, 1312–1319.
- Song, D.; Yang, R.; Fang, S.; Liu, Y.; Long, F. A FRET-based dual-color evanescent wave optical fiber aptasensor for simultaneous fluorometric determination of aflatoxin M1 and ochratoxin A. Microchim. Acta 2018, 185, 508.
- Fang, S.; Song, D.; Zhuo, Y.; Chen, Y.; Zhu, A.; Long, F. Simultaneous and sensitive determination of Escherichia coli O157:H7 and Salmonella Typhimurium using evanescent wave dual-color fluorescence aptasensor based on micro/nano size effect. Biosens. Bioelectron. 2021, 185, 113288.
- Song, D.; Liu, J.; Xu, W.; Han, X.; Wang, H.; Zhuo, Y.; Li, C.; Long, F. On-site rapid and simultaneous detection of acetamiprid and fipronil using a dual-fluorescence lab-on-fiber biosensor. Microchim. Acta 2022, 189, 234.
- Song, D.; Yang, R.; Wang, H.; Fang, S.; Liu, Y.; Long, F.; Zhu, A. Development of dual-color total internal reflection fluorescence biosensor for simultaneous quantitation of two small molecules and their affinity constants with antibodies. Biosens. Bioelectron. 2019, 126, 824–830.
- Song, D.; Yang, R.; Fang, S.; Liu, Y.; Liu, J.; Xu, W.; Long, F.; Zhu, A. A novel dual-color total internal reflection fluorescence detecting platform using compact optical structure and silicon-based photodetector. Talanta 2019, 196, 78–84.
- Liu, Z.; Zhang, W.; Zhang, X.; Wang, S.; Xia, Z.; Guo, X.; Zhao, Y.; Wang, P.; Wang, X.-H. Microstructured optical fiber-enhanced light-matter interaction enables highly sensitive exosome-based liquid biopsy of breast cancer. Anal. Chem. 2023, 95, 1095–1105.
This entry is offline, you can click here to edit this entry!
