Being a controller of cytoprotective actions, inflammation, and mitochondrial function through participating in the regulation of multiple genes in response to stress-inducing endogenous or exogenous stressors, the transcription factor Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) is considered the main cellular defense mechanism to maintain redox balance at cellular and tissue level. While a transient activation of NRF2 protects normal cells under oxidative stress, the hyperactivation of NRF2 in cancer cells may help them to survive and to adapt under oxidative stress. This can be detrimental and related to cancer progression and chemotherapy resistance. Therefore, inhibition of NRF2 activity may be an effective approach for sensitizing cancer cells to anticancer therapy.
1. Introduction
Cells are continuously exposed to exogenous stressors such as pollution, smoke, drugs, xenobiotics, ionizing radiation, and endogenous stressors derived by cellular metabolism. The presence of electrophiles or xenobiotics generates reactive oxygen species (ROS) which can induce alterations in the redox balance and promote cell death by damaging essential macromolecules such as lipids, proteins, and DNA. When ROS production increases or their scavenging by antioxidants decreases, cells undergo a process of oxidative stress [
1,
2]. If present in moderate concentrations, ROS are crucial for cellular life; indeed, they act as a second messengers in the transduction of extracellular signals and in the control of gene expression related to cellular proliferation, differentiation, and survival [
3]. In fact, ROS have double importance in cancer. On the one hand, they can contribute to the occurrence of the modification of normal to cancer cells, on the other hand, a persistent oxidative stress renders cancer cells increasingly vulnerable to additional stressors and reverses drug resistance [
4,
5]. Elevated levels of ROS create conditions appropriate for the promotion of carcinogenesis through various mechanisms including generation of oxidative stress, which may favor oncogene activation, remodeling of tumor-suppressor genes, mitochondrial malfunction, and pro-inflammatory microenvironment [
6,
7]. In addition, cancer cells increase their unique antioxidative capacities [
8,
9,
10,
11]. In this way, cancer cells keep proliferation high and avoid ROS thresholds that would otherwise reduce the survival of cancer cells. In response to excessive ROS production, cancer cells activate several transcriptional programs for maintaining redox homeostasis [
12,
13,
14,
15,
16,
17].
Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) is considered as the principal controller of cytoprotective activities in response to xenobiotics, electrophiles, and oxidants [
5,
18,
19]. NRF2 plays important role in the control of oxidative stress, inflammation, mitochondrial function [
20,
21,
22], and in the regulation of different genes. These genes are encoding antioxidant and Phase II metabolic enzymes and related proteins, linked to antioxidant response system (glutathione synthesis, xenobiotics detoxification, etc.) [
23,
24].
In healthy tissues, a transient activation of the NRF2 signaling pathway prevents cancer initiation by regulating several diverse transcriptional networks. More than 1000 genes possessing an Antioxidant Response Element (ARE) in their promoters can be activated by NRF2 during oxidative stress [
25,
26].
NRF2 is regulated by Kelch-like ECH-associated protein 1 (KEAP1), an adaptor subunit of Cullin 3-based E3 ubiquitin ligase (Cul3). Under normal conditions, KEAP1 binds to NRF2 in the cytoplasm and promotes the ubiquitination and proteasomal degradation of NRF2 and, thus, acts as a negative regulator of NRF2 [
27,
28,
29]. In stress-inducing conditions, KEAP1, a cysteine-rich protein, readily reacts with intracellular ROS or electrophiles and alters NRF2/KEAP1 complex conformation thus inhibiting NRF2 ubiquitylation. Thus, when the interaction between NRF2 and KEAP1 is disrupted, proteasomal degradation of NRF2 decreases, causing de novo NRF2 synthesis within the cell and its increased translocation into the nucleus [
30,
31,
32]. When NRF2 is imported to the nucleus, it heterodimerizes with small Maf proteins (sMaf) and binds to a regulatory enhancer sequence ARE, thus promoting the expression of antioxidant and detoxifying genes and down-modulating the production of pro-inflammatory mediators [
21,
33,
34].
NRF2 is found in almost all cell types and tissues, but it is more abundant in tissues such as the intestine, lung, liver, and kidney, where detoxification reactions occur on site [
35].
Recently, prooncogenic activity of NRF2 in cancer development, cancer progression, cancer cell growth and angiogenesis, and NRF2 involvement in anticancer therapy resistance is also under observation [
2,
36,
37,
38,
39]. While NRF2 defends normal cells from oxidative stress, the excess of NRF2 in cancer cells may help them in adjusting to oxidative stress, surviving and resisting the redox environment (redox adaptation) [
40,
41]. In cancer cells, oncogenic signaling may affect the behavior of NRF2 by increasing its mRNA levels and generating constitutive NRF2 activation. The result is stress adaptation, drug resistance, cells proliferation, and activation of metabolic reprogramming and promotion of the expression of genes related to tumor progression. A cytoprotective role of NRF2 activation has been reported in many diseases associated with oxidative stress; thus, various natural sources (food and medicinal plants) have been tested for their ability to activate NRF2 and for their use as antioxidants and chemopreventive agents [
42,
43]. In many types of cancers, constitutive activation of NRF2 can be detrimental and responsible for cancer progression and chemotherapy resistance [
44]; therefore, inhibition of NRF2 activity may be an effective approach for sensitizing cancer cells to anticancer drugs. Still, there is a scientific debate on whether activation or inhibition of NRF2 might represent an appropriate approach for the prevention and/or treatment of cancer. Regarding NRF2 modulatory effects, its activity as a detoxification agent of anticancer drugs (that possibly leads to chemoresistance) is well known; thus, favorable or adverse repercussions in cancer cells depend on a few factors, such as action control, tumor microenvironment, and cell type [
19].
Among the known inhibitors of NRF2, alkaloids—natural bioactive components—are poorly investigated to date despite their pharmacological potential related to their high pro-oxidant and cytotoxic properties.
Alkaloids are secondary plant metabolites defined by Pelletier as a “cyclic organic compound containing nitrogen in a negative oxidation state which is of limited distribution among living organisms” [
45].
Based on their complex structure and pharmacological properties, different classifications have been proposed. Several physiological pathways are involved in their biosynthesis. Most of them are derived from amino acids (tyrosine, tryptphan, ornithine, lysine, L-aspartate, anthranilic acid, and nicotinic acid), but there are also some classes of alkaloids that have been synthetized from steroids or terpenoids, and they are so-called pseudoalkaloids [
46].
The most widely accepted method of classification of alkaloids is the chemical classification, where the main criterion is the presence of the basic heterocyclic nucleus (i.e., the chemical entity). According to their chemical structure, alkaloids have been grouped based on the nature of the heterocyclic ring in the following groups: mononuclear heterocyclic alkaloids represented as pyrrolidine alkaloids (hygrine) and aporphine alkaloids (boldine); polynuclear heterocyclic alkaloids given as tropane alkaloids (atropine); quinoline alkaloids (quinine); and Rauwolfia alkaloids (reserpine). Non-heterocyclic alkaloids have been found often in plants as atypical alkaloids and they are grouped in the phenylethylamine skeleton (ephedrine and capsaicin), tropolone skeleton (colchicine), and modified diterpene alkaloids (aconitine) [
47].
The diversity in their chemical entity is the basic factor for the wide spectrum of associated pharmacological effects (antibacterial/antiviral activity, antitumor activity, antioxidant, anti-inflammatory, anticholinergic, neurological, and cardiovascular effects) [
48].
2. The Cancer-Preventing Role of NRF2
Numerous studies confirm the regulatory role of NRF2 as a reflection of its cytoprotective action in xenobiotic/oxidative stress. It is reported that this factor regulates the expression of several ARE genes, such as glutathione-S-transferase (GST), heme oxygenase-1 (HO-1), NAD(P)H: quinone oxidoreductase 1 (NQO1), and
γ-glutamylcysteine ligase (
γ-GCS), thus exerting a protective function [
49,
50]. For this reason, it is assumed that by inducing gene expression via NRF2, a detoxification from ROS occurs, which leads to mitigation of the cell oxidative damage.
In addition, NRF2 was also shown to play a role in the response to inflammatory conditions. Namely, NRF2 was able to inhibit the oxidative-stress-derived activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) by decreasing ROS levels. NRF2 also inhibits its nuclear translocation, which in turn leads to a lowered expression of proinflammatory cytokines, as interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and other mediators of inflammation [
21]. Once its work has been completed, NRF2 is degraded in the cytoplasm, therefore allowing the cellular antioxidant defense to return to normal levels.
Indeed, the NRF2-related response to oxidative stress has been proposed in the pathogenesis of various diseases, such as various respiratory diseases [
51], neurodegenerative diseases [
52], inflammatory diseases [
21], cardiovascular diseases [
53], and metabolic diseases [
54]. The NRF2 involvement in various types of cancer was also studied, including breast cancer [
55], prostate cancer [
56,
57], urinary bladder cancer [
58], hepatic carcinoma [
59], pancreatic cancer [
60], non-small cell lung cancer [
61], and colorectal cancer [
62].
The modulation of NRF2 can present an interesting option, both in the prevention and treatment of cancer as well as of other conditions, since its activation can lead to the promotion of antioxidant defense, a reduction in inflammation, detoxification of carcinogens, and inducing the apoptosis of cancer cells.
3. The Cancer-Promoting Role of NRF2 and Its Role in Chemoresistance
NRF2 is involved in increased sensitivity to a number of xenobiotic-induced toxicities and disease pathologies. An overactivation of NRF2 may lead to a negative impact, causing chemoresistance to therapy in cancer cells [
63,
64], thus highlighting the possibility of representing “a double-edged sword” [
65]. The negative role of NRF2 highlighted by the promotion of the growth and survival of various types of cancer cells (NRF2-addicted/activated cancers) has been thoroughly studied and reviewed in the light of a deeper understanding of the mechanisms involved, and to explore the possibility of therapeutic approaches [
66,
67]. In these NRF2-addicted cell types, somatic mutation in NRF2 or its regulatory proteins (KEAP1) are on site, which blocks suppression of NRF2 levels (NRF2 is overexpressed and constitutively activated). These recurrent KEAP1 and NRF2 mutations (and in addition, missense mutations in Cul3) lead to metabolic reprogramming and allow cytoprotective action to cancer cells resulting in enormous proliferation and resistance to anticancer therapy [
68]. Several mechanisms underlying the cancer-promoting role of NRF2 have been revealed (
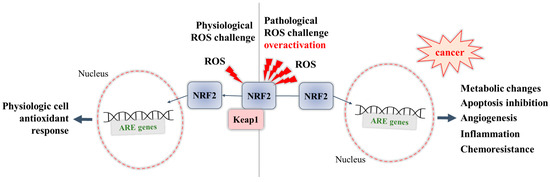
Figure 1):
Figure 1. Dual role of NRF2: during physiological ROS challenge, and during pathological overactivation.
- -
-
Overactivation of NRF2 in an ROS-enriched environment and participation in cancer cell proliferation and tumorigenesis. In various cancers, the cell metabolism increases in order to meet the demands of high cell proliferation, which in turn leads to the generation of ROS that aggravate oxidative stress and disturb the redox balance of cancer cells [
57,
69]. NRF2 is able to adapt itself to operate in a high-oxidizing environment, thus becoming overactivated. The accumulation of NRF2 in cancer cells was shown to promote their survival and proliferation and confer a support to tumorigenesis [
69]. Some studies report that cancer cells can take control of the overactivated NRF2, with this factor even being a key player in the modulation of anabolic metabolism required for cancer cell growth and proliferation [
70].
- -
-
Overexpression of NRF2 resulting in chemoresistance. Early studies have shown that overexpression of NRF2 is responsible for the resistance of cancer cells to chemotherapeutic agents as doxorubicin, cisplatin, and etoposide [
71]. Recent studies have also reviewed the involvement of NRF2 in the activation of multidrug resistance proteins [
72], in particular to modulate the expression of efflux transporters, of phase II metabolic enzymes [
64], and of antiapoptotic genes (Bcl-2) [
73,
74], thus supporting the chemoresistance ability of cancer cells.
This entry is adapted from the peer-reviewed paper 10.3390/ph16060850