Ionic Liquids (ILs) are organic salts that melt commonly below 100 °C, constituted entirely by charged species. The tunability and versatility of ILs have given rise to several applications at the academic and industrial levels. Here the following topics are highlighted: chemical structure; ILs classification according to the possibility of proton transfer, and the historical generations of ILs.
- organic salts
- tunable properties
- intermolecular interaction
1. Introduction
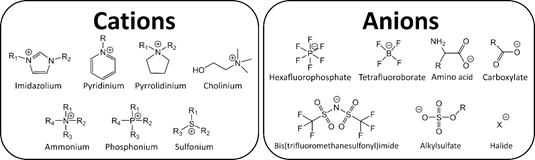
The extensive amount of possible combinations of known cations and anions (106–1018)[1], results in different and unique physicochemical properties such as high thermal stability, large electrochemical window, and low vapor pressure. Because of these attractive properties, ILs have garnered industrial and scientific interest, see Figure 1[2][3].
Figure 1. Typical ions in ionic liquids (ILs).
Imidazolium-based ILs (ImILs) is one of the most popular classes of organic salt explored in the literature. They can be described as a supramolecular polymeric network of intermolecular interactions that determine the interionic distances and consequently the existence of ion pairs and/or aggregates and free volume. The local nanostructural organization and the physicochemical properties of ILs are directly related. The interrelation between transport properties (viscosity, diffusion, conductivity) of IL structure and free volume was reviewed (see link below), analysing the microscopic features to explain and predict macroscopic properties, reaching new perspectives on the properties and application of ILs.
The tunability and versatility of ILs have given rise to several applications such as solvents for synthesis and catalysis[4][5] CO2 capture and storage[6][7][8] energy generation and storage[9], extraction/dissolution of biomass[10][11], and active pharmaceutical ingredients[12][13].
2. Classification
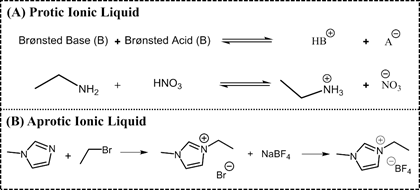
ILs are normally divided into two classes: aprotic ionic liquids (APILs) and protic ionic liquids (PILs). PILs are formed by the transfer of protons from Brønsted acid to Brønsted base, this proton transfer does not take place in APILS due to the nature of the ions constituting the salt. PILs are generally prepared through a neutralization reaction and APILs by a quaternization reaction followed by anion exchange, Figure 2 [14][15].
Figure 2. Typical synthetic route of a protic ionic liquid (PIL) (A) ethylammonium nitrate and an APIL (B) 1-ethyl-3-methylimidazolium tetrafluoroborate.
3. Different Generation of ILs
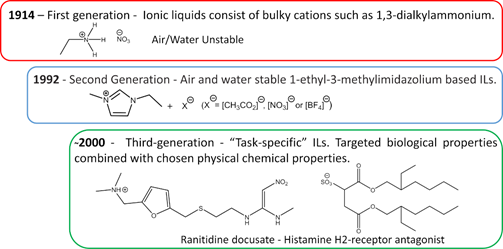
The first IL reported in the literature is a controversial matter. Most authors state that the history starts with Paul Walden’s discovery in 1914 with the report of ethylammonium nitrate ([EtNH3][NO3]) synthesis, see Figure 3[16]; however, some authors attribute the first IL to Gabriel and Weiner in 1888[17], with the synthesis of ethanolammonium nitrate. This confusion arises from the reported melting point, while Walden’s compound was characterized with a melting point of 13–14 °C, Gabriel’s compound exhibited a melting point of 50 °C, substantially higher than room temperature. These works marked the beginning of the First Generation of ILs. Cations of the first generation of ILs are characterized by large volumes, such as 1,3-dialkyl-imidazolium or 1-alkylpyridinium and anions based mostly on halogen aluminate (Al3+)[1][18]. The disadvantage of this generation was the instability in relation to air and water.
Figure 3. Generations of ILs through time.
The introduction of the Second Generation of ILs comes with the preparation of air and water stable ILs, reported in 1992 by Wilkes and Zaworotko[19], based on the 1-ethyl-3-methylimidazolium cation and alternative anions, [CH3CO2]−,[NO3]-, and [BF4]−, see Figure 3. ILs from the second generation are much easier to handle than those of the first generation. It was due to this fact and their particular properties, that IL-based research has become one of the major scientific topics over the last 25 years[20].
During the first decade after the publication of Wilkes and Zaworotko[19], solid scientific background was built on the subject of ILs and specific applications started to be targeted. In Ann Visser’s paper, a series of functionalized imidazolium-based ILs was synthesized to extract heavy metals, Hg2+ and Cd2+ from aqueous solutions[21][22], and the terminology “Task-Specific” was introduced in connection to IL applications. Tuneable physical and chemical properties according to the desired application are what defines the Third Generation of ILs, see Figure 3. Among these properties, rationally selected biological action is already a reality, as evidenced by Hough’s review[23] where several ILs candidates were used as Active Pharmaceutical Ingredients (API) or precursors.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21207745
References
- Natalia V. Plechkova; Kenneth R. Seddon; Applications of ionic liquids in the chemical industry. Chemical Society Reviews 2007, 37, 123-150, 10.1039/b006677j.
- Kohno, Y.; Ohno, H. Ionic liquid/water mixtures: From hostility to conciliation. Chem. Commun. 2012, 48, 7119–7130.
- Beichel, W.; Yu, Y.; Dlubek, G.; Krause-Rehberg, R.; Pionteck, J.; Pfefferkorn, D.; Bulut, S.; Bejan, D.; Friedrich, C.; Krossing, I. Free volume in ionic liquids: A connection of experimentally accessible observables from PALS and PVT experiments with the molecular structure from XRD data. Phys. Chem. Chem. Phys. 2013, 15, 8821.
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576.
- Sheldon, R. Catalytic reactions in ionic liquids. Chem. Commun. 2011, 2399–2407.
- Zhang, X.; Zhang, X.; Dong, H.; Zhao, Z.; Zhang, S.; Huang, Y. Carbon capture with ionic liquids: Overview and progress. Energy Environ. Sci. 2012, 5, 6668.
- Bernard, F.L.; Duczinski, R.B.; Rojas, M.F.; Fialho, M.C.C.; Carreño, L.Á.; Chaban, V.V.; Vecchia, F.D.; Einloft, S. Cellulose based poly(ionic liquids): Tuning cation-anion interaction to improve carbon dioxide sorption. Fuel 2018, 211, 76–86.
- Corvo, M.C.; Sardinha, J.; Casimiro, T.; Marin, G.; Seferin, M.; Einloft, S.; Menezes, S.C.; Dupont, J.; Cabrita, E.J. A Rational Approach to CO2 Capture by Imidazolium Ionic Liquids: Tuning CO2 Solubility by Cation Alkyl Branching. ChemSusChem 2015, 8, 1935–1946.
- Douglas R. Macfarlane; Naoki Tachikawa; Maria Forsyth; Jennifer M. Pringle; Patrick C. Howlett; Gloria D. Elliott; James H. Davis; Masayoshi Watanabe; Patrice Simon; C. Austen Angell; et al. Energy applications of ionic liquids. Energy & Environmental Science 2014, 7, 232-250, 10.1039/c3ee42099j.
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550.
- Passos, H.; Freire, M.G.; Coutinho, J.A.P. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815.
- Ferraz, R.; Branco, L.C.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž. Ionic Liquids as Active Pharmaceutical Ingredients. ChemMedChem 2011, 6, 975–985.
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic Liquids in Pharmaceutical Applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546.
- Lopes, J.N.C.; Rebelo, L.P.N. Ionic liquids and reactive azeotropes: The continuity of the aprotic and protic classes. Phys. Chem. Chem. Phys. 2010, 12, 1948.
- Greaves, T.L.; Drummond, C.J. Protic Ionic Liquids: Properties and Applications. Chem. Rev. 2008, 108, 206–237.
- Walden, P.; Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. St. Petersburg 1914, 1800, 405–422, .
- S. Gabriel; J. Weiner; Ueber einige Abkömmlinge des Propylamins. European Journal of Inorganic Chemistry 1888, 21, 2669-2679, 10.1002/cber.18880210288.
- John S. Wilkes; Joseph A. Levisky; Robert A. Wilson; Charles L. Hussey; Dialkylimidazolium chloroaluminate melts: a new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorganic Chemistry 1982, 21, 1263-1264, 10.1021/ic00133a078.
- John S. Wilkes; Michael J. Zaworotko; Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. Journal of the Chemical Society, Chemical Communications 1992, null, 965-967, 10.1039/c39920000965.
- Matthew D. Morton; Christopher K. Hamer; Ionic liquids – The beginning of the end or the end of the beginning? – A look at the life of ionic liquids through patent claims. Separation and Purification Technology 2018, 196, 3-9, 10.1016/j.seppur.2017.11.023.
- Visser, A.E.; Swatloski, R.P.; Reichert, W.M.; Davis, J.H., Jr.; Rogers, R.D.; Mayton, R.; Sheff, S.; Wierzbicki, A. Task-specific ionic liquids for the extraction of metal ions from aqueous solutions. Chem. Commun. 2001, 135–136.
- Davis, J.H. Task-specific ionic liquids. Chem. Lett. 2004, 33, 1072–1077.
- Whitney L. Hough; Marcin Smiglak; Héctor Rodríguez; Richard P. Swatloski; Scott K. Spear; Daniel T. Daly; Juliusz Pernak; Judith E. Grisel; Richard D. Carliss; Morgan D. Soutullo; et al. The third evolution of ionic liquids: active pharmaceutical ingredients. New Journal of Chemistry 2007, 31, 1429-1436, 10.1039/b706677p.