Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
The tumor microenvironment (TME) plays an important role in the development and progression of hematological malignancies. In addition to several factors, such as growth factors, cytokines, extracellular matrix (ECM) molecules, etc., a growing body of evidence has indicated that extracellular vesicles (EVs) play a crucial role in the communication of tumor cells within the TME, thereby contributing to the pathogenesis of hematological malignancies.
- extracellular vesicles
- tumor microenvironment
- hematological malignancy
- immune cells
- stromal cells
- endothelial cells
- extracellular matrix
- lymphatic system
- biomarker
- drug resistance
1. Introduction
Intercellular communication, an essential biological process of multicellular organisms, is mediated by three different mechanisms: (1) cytoplasmic bridges; (2) direct interactions between adjacent cells via membrane proteins; and (3) cellular secretary molecules [1,2]. Recently, a fourth mechanism has been discovered, which includes the intercellular transfer of extracellular vesicles (EVs) [3,4,5,6,7,8,9,10]. EVs are membrane-bound entities released by almost all types of cells into the extracellular environment [11,12]. EVs are known to transport bioactive molecules, such as proteins, lipids, and nucleic acids in the form of DNA, RNA, miRNA (miR), etc., between cells [13,14]. Detectable levels of EVs are found in nearly all biological fluids, such as blood, urine, synovial fluid, and saliva, and they are even found in the interstitial spaces between cells [12,15,16,17]. Since they are protected from degradation by extracellular proteases and RNases, EVs can be stably stored for long-term use [18]. Depending on their biogenesis, size, release mechanism, content, and function, EVs can be broadly classified into microvesicles, exosomes, and apoptotic bodies (Figure 1) [11,12,19,20].
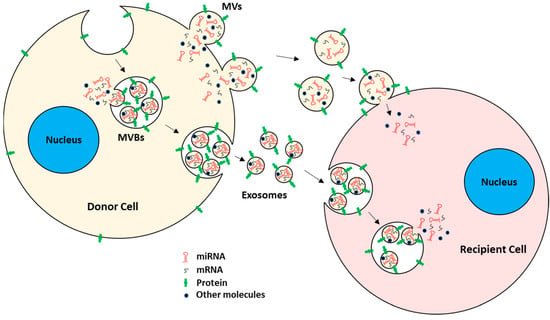
Figure 1. EV biogenesis and uptake by target recipient cells. EVs mainly consist of MVs and exosomes. MVs are generated by plasma membrane outward budding, whereas exosomes are of endocytic origin. Both carry mRNAs, miRNAs, proteins, and other bioactive molecules. EVs are taken up by recipient cells, either by direct fusion with the plasma membrane or by the endocytic pathway. After EV uptake, the EVs’ cargo is released into the recipient cells, hence acting as intercellular communicators between cells.
Microvesicles (MVs). MVs are a type of EV generated by direct outward budding of plasma membrane from cells [13,21]. The formation and release of MVs from cells typically require the interplay of cytoskeletal components, such as actin and microtubules; molecular motors, such as kinesin and myosin; and fusion machinery, such as SNAREs and tethering factors [22,23]. MVs typically ranging from 100 nm to 1 µm in diameter [11,12,15,19,21]. Because MVs are generated from plasma membrane by outward budding, they carry cytosolic and plasma membrane-associated proteins, e.g., proteins clustered at the plasma membrane, such as tetraspanins, which could serve as markers for MVs regardless of the originating cells [24,25]. Other cytoskeletal proteins, such as heat shock proteins, integrins, and proteins containing post-translational modifications, such as glycosylation and phosphorylation, have also been shown to be present in MVs [26,27,28].
Exosomes. Exosomes are the other subtype of the EV of endocytic origin [15,29] with a typical diameter of 30 to 150 nm [30,31]. Specifically, exosomes are formed by inward budding of early endosomal membrane, which matures into multivesicular bodies (MVBs) [12,15,32]. MVBs eventually fuse with the plasma membrane, releasing their content of exosomes into the extracellular space [32,33,34,35,36]. The regulation of MVBs and the formation and subsequent release of exosomes are mediated by the endosomal sorting complexes required for transport (ESCRT) pathway [37,38,39]. Since exosome generation is mediated by the ESCRT pathway, ESCRT and its accessory proteins (Alix, TSG101, HSC70, and HSP90β) are believed to be present in all exosomes regardless of the type of originating cells and hence serve as exosome markers [40,41,42,43,44]. Other than the ESCRT pathway, exosome generation is also thought to be dependent on sphingomyelinase enzymes since, in some instances, cells with ESCRT deficiency also produce significant numbers of CD63+ exosomes [45,46,47,48]. Both exosomes and MVs have been shown to participate actively in cell–cell communication, maintenance of cells, and tumor progression by transporting their cargo between cells [49,50]. EVs are readily taken up by the recipient cells, either by direct fusion with the plasma membrane or by fusion with the endosomal membrane after endocytosis [51,52,53,54].
Apoptotic bodies. Apoptotic bodies, which are in general not considered to be a true form of EV, are larger, ranging from 50 nm to 5 µm in diameter [55,56,57], and are released from cells undergoing programmed cell death [19,56]. These bodies are generated by the separation of plasma membrane from the cytoskeleton due to enhanced hydrostatic pressure during contraction of cells [58,59]. In contrast with MVs and exosomes, apoptotic bodies contain cellular organelles, chromatin, and a few glycosylated proteins [19,42,60,61]. Hence, higher levels of nuclear proteins (such as histones), mitochondrial proteins (such as HSP60), Golgi, and endoplasmic reticulum-associated proteins (such as GRP78) are expected to be observed in apoptotic bodies.
2. Tumor-Derived EVs
Tumor-derived EVs are distinguished from normal cell-secreted EVs due to the presence of unique tumor-specific ‘labels’ [62,63,64,65]. Tumor-derived EVs have been shown to carry oncogenic proteins or nucleic acids (such as DNA, RNA, miRNAs, etc.) which facilitate tumor progression. Oncogenic bioactive molecules are enriched in tumor-derived EVs compared to normal cell-derived EVs [66,67,68]. For example, chromosome segregation 1 like protein (CSE1L), a transmembrane protein, is enriched in tumor-derived EVs, not only triggering Ras-dependent EVs biogenesis but also promoting metastasis of B16F10 and melanoma cells [69]. Adriamycin-resistant breast cancer cell-derived EVs were shown to carry transient receptor potential cation channel subfamily C member 5 (TrpC5) and to transfer of EV. TrpC5 confers endothelial cell resistance against chemotherapeutic regimens [70]. On the other hand, the transfer of oncogenic nucleic acids such as miRNAs, specifically miR-221 from highly aggressive breast tumor cells to nonaggressive cancer cells, via EVs, contributing to the promotion of epithelial-to-mesenchymal transition (EMT) [71] and leading to the induction of proliferation and metastasis while preventing drug-induced apoptosis of EVs’ fused recipient cells [5]. Therefore, tumor cells more often induce oncogenic transformations into normal healthy cells via the transfer of oncogenic bioactive molecules through EVs [72,73].
3. Hematological Malignancies
Hematological malignancies are defined as tumors that commence in blood-forming tissues, such as bone marrow or cells of the immune system, resulting in leukemias, lymphomas, and myelomas [74,75]. Hematological malignancies are considered to be among the leading causes of cancer-related deaths worldwide [76,77,78]. In the United States itself, an estimated 184,710 new cases of hematological neoplasms were reported with 57,380 deaths in 2023, and the incidence increases with age [79]. GLOBOCAN 2020 reported non-Hodgkin lymphoma to be the predominant hematological cancer worldwide with 544,352 new cases and 259,793 deaths, followed by leukemia, with 474,519 new cases and 311,594 deaths worldwide [80]. The outbreak of COVID-19 further increased the death rate of patients suffering from various hematological malignancies [81,82,83,84,85].
EVs are known to play an important role in cell–cell communication via the transfer of bioactive cargo molecules. However, the role of EVs in the crosstalk of tumor cells with cells in the tumor microenvironment (TME) and other distant cells remains to be completely determined in the context of the pathogenesis of hematological malignancies.
4. EVs in Cell-Cell and Cell-Extracellular Matrix Communication in the TME
The TME is the environment surrounding the tumor cells in the body [89,90]. It consists of immune cells, stromal cells, fibroblasts, extracellular matrix (ECM), and cells of the blood and lymphatic vessels [89,90]. Tumor cells and their TME are in constant interaction, thereby regulating each other either positively or negatively [91]. Dynamic interaction between cancer cells and TME components not only supports tumor growth and development [92,93] but also promotes local invasion and metastatic dissemination of cancer [94,95]. In hypoxic and acidic conditions, the TME often promotes angiogenesis, a process of restoring nutrient and oxygen supply, as well as removing metabolic waste [96,97,98]. Additionally, the infiltration of various immune cells into the TME performs various pro- and anti-tumorigenic functions [99,100,101,102]. EVs play an important role in intercellular communication via the transfer of bioactive cargo molecules between cells [103]. Tumor-derived EVs also act as a communicating vehicle between cancer cells and cells in the TME and in some instances also with distant cells. On the other hand, cells in the TME often release EVs that interact with tumor cells, influencing tumor development and progression.
Effects of hematological malignancy-derived EVs on immune cells. Immune cells play a major role in the elimination of tumors through diverse mechanisms, and the evasion of immune surveillance serves as an important step for developing tumor niches and successful establishment of tumors. Immune evasion, a strategy facilitated by tumor-derived EVs is utilized in different ways to target various immune cells (Figure 2). Tumor-derived EVs, through their receptor-mediated uptake, can introduce several suppressive factors, e.g., miRNA, DNA, pro-apoptotic factors, metabolites, and various enzymes, into immune cells (Table 1). They can also alter the activation of immune cells through inhibitory cell surface receptors [104,105,106,107].
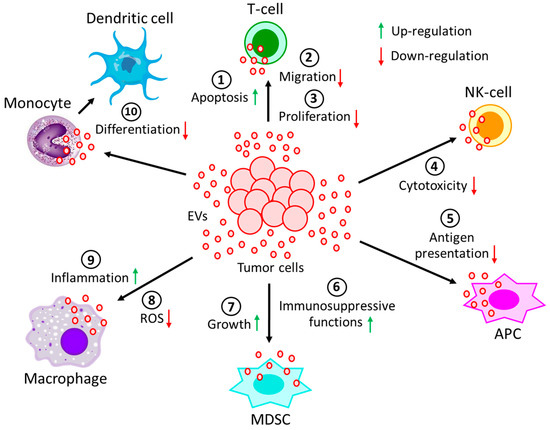
Figure 2. Effect of EVs generated during hematological malignancies on different immune cells. EVs, derived from tumor cells not only (1) induce apoptosis of T-cells, but also reduce T-cell (2) migration and (3) proliferation. Moreover, tumor-derived EVs (4) decrease the cytotoxicity of NK-cells and (5) restrain the processing of antigen by APCs. On the other hand, tumor-derived EVs (6) induce immunosuppressive functions of MDSCs, as well as (7) promote MDSCs growth. Again, tumor-derived EVs (8) not only prevent the generation of ROS in macrophages but also (9) promote macrophages’ pro-inflammatory response. (10) The differentiation of monocytes into dendritic cells is often perturbed by the incorporation of tumor-derived EVs into monocytes. All of these processes contribute to the development and progression of the tumor.
In activated T-cells, tumor-derived EVs induce the down-regulation of CD3ζ and JAK3 expression via transcriptional regulation, thereby facilitating Fas/FasL-mediated apoptosis of CD8(+) T-cells [108]. Chronic lymphocytic leukemia (CLL)-derived EVs were shown to down-regulate CD69 expression in T-cells via miR-363 transfer, thereby affecting effector T-cell migration [109,110,111]. Tumor cells are often shown to evade the host immune system via the activation of the PD-L1/PD-1 pathway. PD-L1 is expressed on the surfaces of various tumor cells, whereas its receptor, PD-1, is present on T-cells. PD-L1 binding to PD-1 results in the apoptosis of T-cells, thereby evading host immune responses. The upregulation of PD-1 has been observed in various T-cell populations after exposure to diffuse large B cell lymphoma (DLBCL)-derived EVs [112]. EVs released from B-cell lymphoma (BCL) under chemotherapy are enriched with CD39 and CD73, and they hydrolyze ATP, which is generated from chemotherapy-treated tumor cells and transformed into adenosine [113], which in turn affects the immune system by inhibiting T-cell activity and proliferation [114].
Table 1. The effect of EVs, derived from hematological malignancy on immune cell function.
| Originating Cells | Effector Cells | Malignancies | Functions | References |
|---|---|---|---|---|
| Leukemic cells | CD8(+)T-cells | Solid tumors or AML | Down-regulates CD3ζ and JAK3 expression and promotes Fas/FasL-mediated T-cell apoptosis | [108] |
| Leukemic cells | CD4(+)T-cells | CLL | Down-regulates CD69 expression via miR-363 transfer and affects effector T-cell migration | [109,110,111] |
| Lymphoma cells | T-cells | DLBCL | Induces PD-1 expression in T-cells and enhances T-cell apoptosis | [112] |
| Lymphoma cells | T-cells | BCL | Carries CD39 and CD73 and hydrolyzes ATP to generate adenosine to inhibit T-cell activity and proliferation | [114] |
| Lymphoma cells | NK-cells, APCs | BCL, TCL | Carries MHC, APO2L, FASL, TCR, and NKG2D and inhibits NK-cells cytotoxicity and antigen processing of APCs | [115,116,117] |
| Non-leukemic cells | NK-cells | CLL | Carries BAG6 and activates NK-cells, but activated NK-cells are eliminated by lymphocytes | [118] |
| Myeloma cells | MDSCs, | MM | Induces growth and immunosuppressive activity | [119,120] |
| NK-cells, | Reduces NK-cells’ cytotoxicity | [121] | ||
| Immune cells | Carries ectoenzyme, CD38, which converts nucleotides into adenosine to suppress immune system | [122] | ||
| Leukemic cells | NK-cells | AML | EVs’-bound TGFβ1 reduces NK-cells’ cytotoxicity | [123] |
| Lymphoma cells | Monocytes | - | Releases TNF-α, IL-1β, and IL-6 and prevents monocyte differentiation into dendritic cells | [124] |
| Lymphoma cells | Macrophages | DLBCL | Transfers MyD88 and stimulates pro-inflammatory NF-κB signaling pathway | [125] |
| Leukemic cells | Macrophages | CML | Polarizes macrophages to M2-phenotype to induce TNF-α and IL-10 expression and down-regulates NO and ROS generation | [126] |
| Tumor cells | Neutrophils | CAT | Promotes NET formation, reduced generation of suppressor cells | [127,128,129] |
| Lymphoma cells | MDSCs | Lymphoma | Carries HSP72 and promotes suppressive functions | [124] |
Abbreviations of malignancies: AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B cell lymphoma; BCL, B-cell lymphoma; TCL, T-cell lymphoma; MM, multiple myeloma; CAT, cancer associated thrombosis; CML, chronic myelogenous leukemia.
EVs are known to carry surface receptors of the originating cells. For example, EVs from B- and T-cell lymphomas are capable of carrying cell surface molecules, such as major histocompatibility complex (MHC), Apo2 ligand (APO2L), Fas ligand (FASL), T-cell receptor (TCR), and natural-killer group-2 member-D (NKG2D), which not only inhibit the cytotoxicity of NK-cells, thereby promoting T-cell apoptosis, but also down-regulate the processing of antigens by antigen presenting cells (APCs) [115,116,117]. The plasma soluble ligand, BAG6, in CLL patients binds to the receptor NKp30 on NK-cells, causing NK-cell inactivation [118]. In contrast, BAG6, carried by the EVs, activates NK cells, which have the ability to kill tumor cells [118]. Hence, the dysregulated balance between the soluble form of BAG6 and its EVs’ bound form determines the immune evasion of CLL. EVs from multiple myeloma (MM) cells suppress the immune system through different mechanisms. First, MM-derived EVs enhance the growth and immunosuppressive activity of myeloid-derived suppressor cells (MDSCs) in both in vitro and in vivo MM xenograft murine models [119,120]. Second, EVs from MM cells reduce NK-cells’ cytotoxic activity [121]. Third, the ectoenzyme, CD38, on MM-derived EVs converts nucleotides into adenosine, which is a well-known suppressor of the immune system [122].Finally, CD38-positive EVs were shown to be internalized by FcR-positive cells, such as monocytes, MDSCs, and NK cells, after binding to an anti-CD38 mAb (daratumumab), although the effects are still under investigation [123]. TGFβ1, on the other hand, was shown to play an important role in immune-evasive mechanisms of leukemic EVs [130]. EVs from the sera of acute myeloid leukemia (AML) patients are enriched with membrane-bound TGFβ1, which significantly reduces the killing properties of NK-cells [131]. Moreover, TGFβ1 on chronic myelogenous leukemia (CML) EVs stimulates the proliferation and colony formation of CML cells [130].
Monocytes are the highly dynamic cells differentiated into macrophages and dendritic cells to effectively protect the body from tumor assault. The fusion of lymphoma-derived EVs with monocytes results in the release of TNF-α, IL-1β, and IL-6, which in turn impair monocytic differentiation into dendritic cells [123]. DLBCL-derived EVs readily transfer MyD88 to the macrophages, thereby stimulating the pro-inflammatory signaling pathway, NF-κB, independent of TLR and IL-1R activation [125]. Similarly, CML-derived EVs have been demonstrated to alter the macrophage polarization to a more M2 phenotype within the tumor microenvironment, hence demonstrating up-regulation of IL-10 and TNF-α expression while down-regulating macrophage NO and ROS generation [126]. More often, in a tumor environment, M2 macrophages are converted into tumor-associated macrophages (TAMs) with the potential of releasing pro-tumorigenic growth factors, cytokines, and chemokines to enhance tumor progression [132,133,134,135].
Like monocytes, granulocytes, such as neutrophils, are highly plastic cells that can be readily influenced by tumor-derived EVs. Tumor-derived EV-treated neutrophils have been shown to promote NET formation and reduce the generation of suppressor cells, which are beneficial for tumor progression [127,128,129].
MDSCs, the primary cells associated with reducing the tumorigenicity of T-cells and NK-cells, have been shown to be modulated by heat shock proteins (HSPs) inside the EVs, such as HSP70 and HSP72 [124,136,137].
Crosstalk between tumor cells and stromal cells through EVs. Stromal cells refer to a highly heterogenous population of cells that provide structural and physiological support for hematopoietic cells. In cancer, stromal cells often contribute to disease progression by supporting growth, development, and metastasis of tumors (Figure 3). Table 2 also summarizes interactions between tumor cells with cells in the TME via the release of EVs.
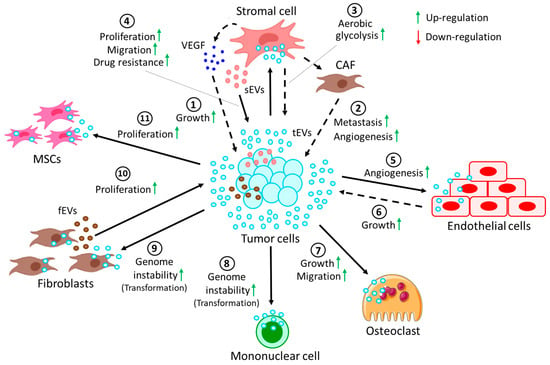
Figure 3. Crosstalk between tumor cells and cells in the TME through EVs. (1) EVs derived from tumor cells (tEVs) target stromal cells to release VEGF, which in turn induces growth of the tumor. tEVs induce the conversion of stromal cells into CAFs, which promote (2) tumor metastasis and angiogenesis. Moreover, tEV-fused stromal cells (3) induce the rate of aerobic glycolysis in tumor cells. On the other hand, (4) stromal cell-derived EVs (sEVs) promote proliferation and migration and confer drug resistance to tumor cells. (5) tEVs, upon fusion with endothelial cells, often promote angiogenesis, whereas (6) tEV-fused endothelial cells in turn contribute to tumor growth. (7) tEVs also promote the growth and migration of osteoclasts. More often, tEVs are shown to induce genomic instability in (8) several mononuclear cells and (9) fibroblasts, resulting in their transformation into pro-cancerous cells. (10) On the other hand, fibroblast-derived EVs (fEVs) induce the proliferation of the tumor. (11) Moreover, tEVs are also shown to induce the proliferation of MSCs, which in turn contribute to the progression of the tumor.
In CLL patients, activation of the tumor microenvironment, thereby promoting disease progression, is favored by tumor cell-secreted EVs [138]. CLL-derived EVs activate the AKT survival pathway in stromal cells, in turn releasing vascular endothelial growth factor (VEGF), thereby contributing to tumor survival [139]. Again, CLL-derived EVs facilitate the conversion of stromal cells into cancer-associated fibroblasts (CAFs), promoting tumor metastasis and angiogenesis [140,141].
In acute lymphoblastic leukemia (ALL), EV-mediated transfer of galectin 3 (GAL3) from stromal cells to ALL cells stimulates endogenous GAL3 expression, which confers protection against drug treatment [142]. On the other hand, EVs derived from ALL cells induce a metabolic shift from oxidative phosphorylation to aerobic glycolysis in stromal cells [143].
Table 2. Tumor cells communicate with the stromal cells and vice versa via EVs transfer.
| Originating Cells | Effector Cells | Malignancies | Functions | References |
|---|---|---|---|---|
| Tumor cells | Stromal cells | CLL | Tumor-derived EVs induce stromal cells to release VEGF to promote tumor survival | [139] |
| Tumor cells | Stromal cells | CLL | Tumor-derived EVs convert stromal cells into CAFs, thereby promoting metastasis and angiogenesis | [140,141] |
| Stromal cells | Cancer cells | ALL | Stromal cell-derived EVs induce GAL3 expression in cancer cells, hence inducing drug resistance | [142] |
| Tumor cells | Stromal cells | ALL | Switch oxidative phosphorylation to aerobic glycolysis in favor of cancer progression | [143] |
| Stromal cells | Tumor cells | MM | Stromal cells from MM induce proliferation, migration, and survival of tumor cells | [144] |
| Tumor cells | Endothelial cells | MM | Tumor cell-derived EVs in hypoxic conditions affect miR-135b, targeting HIF-1 pathway to promote angiogenesis | [145] |
| Tumor cells | Endothelial cells | MM | Tumor cell-secreted EVs activate endothelial STAT3 pathway, which promotes angiogenesis and tumor growth | [146] |
| Tumor cells | Osteoclasts | MM | MM-derived EVs support osteoclast growth and migration | [147] |
| Fibroblasts | Tumor cells | MM | EVs carry clBcl-xL, which helps in EV uptake by the tumor cells to promote tumor proliferation | [148] |
| Tumor cells | MSCs | ATL | Tumor cell-derived EVs transfer miR-155 and miR-21 to the MSCs, thereby inducing MSC proliferation | [149] |
| Leukemic CD34+ cells | MSCs | AML | AML CD34+ cell-derived EVs reduce further development of CD34+ cells from MSCs via miR-7977 | [150] |
| MSCs | CD34+ cells | MPN | EVs carry miR-155 from MSCs, increasing granulocyte CFU numbers in neoplastic CD34+ cells | [151] |
| Leukemic cells | MSCs | CML | CML-derived EVs induce IL-8 release from MSCs, thereby promoting CML survival | [152] |
| BCR-ABL + tumor cells | Mononuclear | CML | Induce genome instability, leading to malignant transformation of cells | [153] |
| Tumor cells | Fibroblasts | TCL, CML | Transfer of hTERT mRNA via the EVs results in induced hTERT expression in the fibroblasts, leading to genome instability | [154,155] |
Abbreviations of malignancies: CLL, chronic lymphocytic leukemia; ALL, acute lymphocytic leukemia; MM, multiple myeloma; ATL, adult T-cell leukemia/lymphoma; AML, acute myeloid leukemia; MPN, myeloproliferative neoplasm; CML, chronic myelogenous leukemia, TCL, T-cell leukemia.
In the case of MM, stromal cell-derived EVs from MM induce proliferation, migration, and survival of tumor cells, whereas normal stromal cell-secreted EVs prevent tumor proliferation [144]. Under hypoxic conditions, MM-derived EVs were shown to affect miR-135b, which targets the HIF-1 pathway to induce tumor angiogenesis [145]. In another study, MM-derived EVs were reported to modulate the STAT3 pathway in endothelial cells, not only promoting angiogenesis but also inducing tumor growth via the release of VEGF and IL-6, respectively [146]. Moreover, EVs from MM are also capable of inducing growth and migration of osteoclasts (OCs), thus promoting the development of bone diseases in MM patients [147]. Stromal fibroblast-derived EVs actively carry Bcl-xL and its cleaved counterpart, clBcl-xL, facilitating the uptake of EVs by MM cells, hence promoting tumor proliferation [148].
Tumor cells from adult T-cell leukemia/lymphoma (ATL) often release miR-155 and miR-21 through EVs, triggering mesenchymal stem cell (MSC) proliferation and aiding the development of a friendly environment for leukemic progression [149]. Another study indicated that AML CD34+ cell-derived EVs efficiently transfer miR-7977 to MSCs to reduce proliferation of CD34+ cells [150].
miR-155 is found to be selectively packaged in the EVs of MSCs from myeloproliferative neoplasms (MPNs), increasing granulocyte CFU numbers in neoplastic CD34+ cells [151]. Moreover, leukemic cell survival in CML is enhanced by IL-8 production from MSCs upon incorporation of CML cell-derived EVs [152]. Another study showed that EVs from BCR-ABL+ CML tumor cells induced genomic instability in normal mononuclear cells, leading to malignant transformations [153]. Additionally, hTERT mRNA is known to be transported from TCL and CML cells to fibroblasts via EVs, leading to ectopic hTERT expression in fibroblasts [154] and a resultant switch to a tumor-like phenotype [155].
Interaction of EVs with the endothelium in the context of hematological malignancies. Hematological neoplasm-derived EVs are believed to communicate with the cells of the endothelium and vice versa. Hematological tumors, endowed with angiogenic-promoting ability, are dependent on the vascular endothelium for growth, migration, and invasion [156]. More often, tumor-EVs have been shown to activate the endothelial cells, contributing to tumor angiogenesis, whereas endothelial EVs in the TME induce tumor cells to remodel ECM components, thereby providing supplements for the growth of the tumor [157]. For example, EV-mediated transfer of VEGF and VEGF receptor from AML to endothelial cells promotes endothelial glycolysis, leading to vascular remodeling and chemoresistance [158,159]. Multiple signaling pathways appear to be regulated in MM, leading to increased viability of a bone marrow endothelial cell line (STR10), enhanced, angiogenesis and immunosuppression, further facilitating the progression of MM [120,159]. Piwi-interacting RNA-823 (piRNA-823), essentially carried by MM-derived EVs, transforms the endothelial cells, in turn promoting MM growth [159,160]. However, treatment of MM with a protease inhibitor, bortezomib, bestowed anti-angiogenic properties on MM-derived EVs [161]. CML-derived EVs induce the expression of VCAM-1 and IL-8 in endothelial cells, thereby promoting angiogenesis [162]. However, pretreatment of CML cells with curcumin decreases endothelial proliferation and migration via the release of miR-21-enriched EVs [163]. Moreover, CML-EVs, packaged with miR-126, have been found to negatively regulate the expression of VCAM-1 and CXCL12 in endothelial cells, thereby restricting CML motility and adhesion [159,164]. In CLL, tumor-derived EVs are shown to up-regulate the expression of ICAM-1, CXCL1, and IL-34 in endothelial cells, leading to angiogenesis and CLL proliferation [140]. In PML, EVs carry a significant amount of retinoic acid receptor-α (RAR-α), and the transfer of PML-EVs’ RAR-α to the endothelial cells results in the acquisition of tissue factor, hence imparting pro-coagulant properties to the endothelial cells [159,165].
Interaction of EVs with extracellular matrix (ECM). ECM, the physical scaffold, plays a pivotal role not only in the communication of cells with nearby cells but also in the growth, function, and movement of cells [166,167,168]. Tumor cells often communicate with the ECM via the release of EVs [169,170]. For example, surface heparan sulfate (sHS)-positive EVs from MM cells bind to one ECM component, fibronectin, thereby acting as a ligand for sHS-positive target cells. The binding of EVs’ fibronectin with sHS-positive cells triggers the activation of MAPKs (p38 and ERK1/2), resulting in the production of MMP-9 and DKK1, which essentially regulate the invasion of MM cells [171]. Cancer cell-derived EVs often induce the secretion of EMMPRIN, MMP-9, and IL-6 from human monocytic cell lines, thereby modulating the ECM to promote migration and inflammation, ultimately leading to tumor progression [172]. CD30+ EVs have often been shown to stick to long actin or tubulin protrusions of HL cells grown in 3D-matrigel or tissues, which could be a guiding mechanism of EVs to reach distant cells, thereby enabling cell–cell communication [173]. Figure 4 illustrates the contribution of EVs to the progression of hematological tumors by interacting with the ECM.
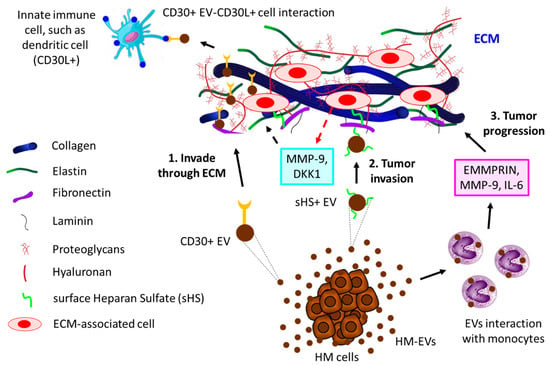
Figure 4. The interaction of HM-derived EVs with ECM leads to tumor progression. 1. HL-derived CD30+ EVs invade through ECM to reach distant innate immune cells (here shown is a dendritic cell, for example) that are positive for CD30 ligand (CD30L+), leading to tumor cell–immune cell interaction. 2. HM cells, such as MM-released sHS+ EVs, interact with ECM fibronectin, which further interacts with other ECM cells, thereby releasing MMP-9 and DKK1, which contribute to MM invasion through ECM. 3. HC-derived EVs induce monocytes to secrete EMMPRIN, MMP-9, and IL-6, which not only promote tumor invasion but also influence tumor inflammation, ultimately leading to tumor progression through ECM.
EVs in lymphatic malignancies. Lymphoma is a hematological malignancy associated with the lymphatic system [174,175]. A growing body of evidence has indicated that EVs actively participate in the pathogenesis of various lymphomas [176,177]. The incorporation of EVs, generated from EBV-associated lymphomas into monocytes or macrophages, transforms the immune regulatory mechanisms, leading to tumor evasion [178]. Lymphoma-derived EVs have often been shown to promote the angiogenic process by delivering angiogenic mRNA, miRNA, and proteins such as VEGF [179]. EBV lymphoma-induced macrophages have been shown to release secreted phospholipase A2 of group X (sPLA2-X), which hydrolyzes lymphoma-derived EVs’ phospholipids, thereby allowing for better uptake of EVs and associated lipid mediator signaling in TAMs, contributing to lymphoma growth [180]. HL-derived EVs were demonstrated to be internalized by TME fibroblasts and promote the release of pro-inflammatory cytokines, growth factors, and angiogenic factors, which together contribute to growth of lymphomas [181]. Moreover, HL-derived large EVs were shown to promote the release of IL-1β from monocytes depending on CD44 transfer, whereas both large and small HL-EVs confer immunomodulatory effects through eATP [182]. A recent study indicated that plasma EVs of pediatric HL can be used as a potential biomarker for relapse occurrence of HL [183]. DLBCL-derived EVs were found to be taken up by the tonsillar cells and stromal cells, which contribute to the progression of DLBCL [184]. Moreover, the PD-L1+ EV population was shown to be elevated in the plasma of DLBCL patients and could serve as a biomarker for DLBCL [185]. A recent study also indicated that DLBCL-tumor-derived EVs carry miR-125b-5p, which is readily taken up by DLBCL cells and reduces DLBCL sensitivity to rituximab via miR-125b-5p-mediated targeting of TNFAIP3, reflecting the ability of DLBCL-EVs to influence other cells in the TME [186]. Burkitt lymphoma-derived EVs not only inhibit autophagy and apoptosis but also promote lymphoma growth via miR-106a-mediated targeting of Beclin1 [187].
This entry is adapted from the peer-reviewed paper 10.3390/biom13060897
This entry is offline, you can click here to edit this entry!
