Phenolic compounds are classified as primary antioxidants and originate from one of the main classes of secondary metabolites in plants. They have antioxidant properties through several mechanisms: (i) the ability to remove free radicals and inhibit the formation of reactive species during the normal course of metabolism; (ii) preventing the occurrence of damage to lipids, proteins, and nucleic acids; and (iii) preventing consequent cell damage and death. Thus, they are commonly associated with preventing the development of cardiovascular diseases, neurodegenerative diseases, autoimmune diseases, diabetes, and cancer.
- nutraceutical properties
- human health
- phenolic compounds
1. Natural Phenolic Antioxidants in Beverages
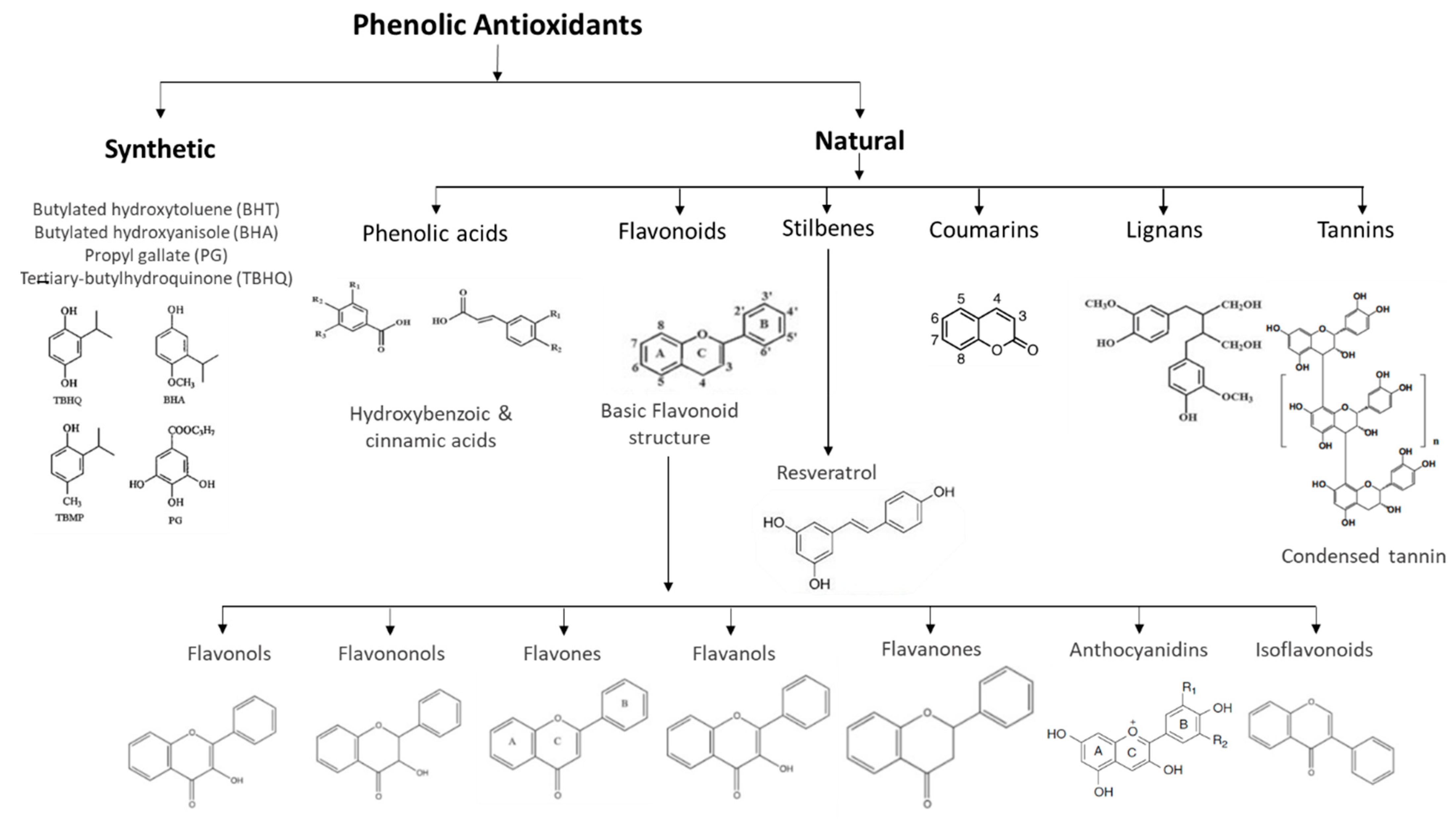
2. Synthetic Phenolic Antioxidants in Beverages
This entry is adapted from the peer-reviewed paper 10.3390/beverages7010012
References
- Sikora, E.; Cieślik, E.; Topolska, K. The sources of natural antioxidants. Acta Sci. Pol. Technol. Aliment. 2008, 7, 5–17.
- Jayaprakasha, G.; Singh, R.; Sakariah, K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290.
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399.
- Massini, L.; Rico, D.; Martín-Diana, A.B.; Barry-Ryan, C. Quality Markers of Functional Tomato Juice with Added Apple Phenolic Antioxidants. Beverages 2016, 2, 4.
- Berdahl, D.R.; Nahas, R.I.; Barren, J.P. Synthetic, and natural antioxidant additives in food stabilization: Current applications and future research. In Oxidation in Foods and Beverages and Antioxidant Applications; Decker, E.A., Elias, R.J., McClements, D.J., Eds.; Woodhead Publishing: Cambridge, UK, 2010; Volume 1, pp. 272–313.
- Liu, R.; Mabury, S.A. Synthetic Phenolic Antioxidants: A Review of Environmental Occurrence, Fate, Human Exposure, and Toxicity. Environ. Sci. Technol. 2020, 54, 11706–11719.
- Dragovicuzelac, V.; Levaj, B.; Mrkic, V.; Bursac, D.; Boras, M. The Content of Polyphenols and Carotenoids in Three Apricot Cultivars Depending on Stage of Maturity and Geographical Region. Food Chem. 2007, 102, 966–975.
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699.
- Fernández-Mar, M.I.; Mateos, R.; Garcıa-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813.
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell Longev. 2015, 837042.
- Gea, A.; Sánchez-Tainta, A. Red Wine Moderate Consumption and at Mealtimes. In Prevention of Cardiovascular Disease through the Mediterranean Diet; Sánchez-Villegas, A., Sánchez-Tainta, A., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 151–157.
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897.
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214.
- Fiori, L.; de Faveri, D.; Casazza, A.A.; Perego, P. Grape by-products: Extraction of polyphenolic compounds using supercritical CO2 and liquid organic solvent—A preliminary investigation Subproductos de la uva: Extraccion de compuestos polifenolicos usando CO2 supercritico y disolventes organicos liquidos—Una investigacion preliminar. CyTA J. Food 2009, 7, 163–171.
- Obreque-Slier, E.; Peńa-Neira, A.; López-Solís, R.; Zamora-Marín, F.; Ricardo-da Silva, J.M.; Laureano, O. Comparative study of the phenolic composition of seeds and skins from Carménčre and Cabernet Sauvignon grape varieties (Vitis vinifera L.) during ripening. J. Agric Food Chem. 2010, 58, 3591–3599.
- Giuffrč, A.M. HPLC-DAD detection of changes in phenol content of red berry skins during grape ripening. Eur. Food Res. Technol. 2013, 237, 555–564.
- Budić-Leto, I.; Lovrić, T.; Pezo, I.; Gajdoš Kljusurić, J. Study of dynamics of polyphenol extraction during traditional and advanced maceration processes of the Babić grape variety. Food Technol. Biotechnol. 2005, 43, 47–53.
- Klenar, I.; Berović, M.; Wondra, M. Phenolic compounds from the fermentation of cultivars Cabernet Sauvignon and Merlot from the Slovenian coastal region. Food Technol. Biotechnol. 2004, 42, 11–17.
- Vilela, A. The Importance of Yeasts on Fermentation Quality and Human Health-Promoting Compounds. Fermentation 2019, 5, 46.
- Coletta, A.; Berto, S.; Crupi, P.; Cravero, M.C.; Tamborra, P.; Antonacci, D.; Daniele, P.G.; Prenesti, E. Effect of viticulture practices on concentration of polyphenolic compounds and total antioxidant capacity of Southern Italy red wines. Food Chem. 2014, 152, 467–474.
- Cordova, A.C.; Sumpio, B.E. Polyphenols are medicine: Is it time to prescribe red wine for our patients? Int. J. Angiol. 2009, 18, 111–117.
- Soni, R.P.; Katoch, M.; Kumar, A.; Ladohiya, R.; Verma, P. Tea: Production, Composition, Consumption and it’s Potential as an Antioxidant and Antimicrobial Agent. Int. J. Food. Ferment. Technol. 2015, 5, 95–106.
- Vilela, A.; Pinto, T. Grape Infusions: The Flavor of Grapes and Health-Promoting Compounds in Your Tea Cup. Beverages 2019, 5, 48.
- McCarthy, T.L.; Kerry, J.P.; Kerry, J.F.; Lynch, P.B.; Buckley, D.J. Evaluation of the antioxidant potential of natural food/plant extracts as compared with synthetic antioxidants and vitamin E in raw and cooked pork patties. Meat Sci. 2001, 58, 45–52.
- Bártíková, H.; Skálová, L.; Valentova, K.; Matoušková, P.; Szotáková, B.; Martin, J.; Kvita, V.; Boušová, I. Efect of oral administration of green tea extract in various dosage schemes on oxidative stress status of mice in vivo. Acta Pharm. 2015, 65, 65–73.
- Cacho, J.I.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Determination of synthetic phenolic antioxidants in soft drinks by stir-bar sorptive extraction coupled to gas chromatography-mass spectrometry. Food Addit. Contam. Part A 2015, 32, 665–673.
- Makahleh, A.; Saad, B.; Bari, M.F. Synthetic phenolics as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2015; pp. 51–78.
- Furia, T.E. CRC Handbook of Food Additives, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1980; Volume 2, p. 424. ISBN 0849305438.
- Nieva-Echevarría, B.; Manzanos, M.J.; Goicoechea, E.; Guillén, M.D. 2,6-Di-tert-butyl-hydroxytoluene and its metabolites in foods. Compr. Rev. Food Sci. Food Saf. 2015, 14, 67–80.
- Lanigan, R.S.; Yamarik, T.A. Final report on the safety assessment of BHT. Int. J. Toxicol. 2002, 21 (Suppl. 2), 19–94.
- Dolatabadi, J.E.N.; Kashanian, S. A review on DNA interaction with synthetic phenolic food additives. Food Res. Int. 2010, 43, 1223–1230.
- Lavagnini, I.; Urbani, A.; Magno, F. Overall calibration procedure via a statistically based matrix-comprehensive approach in the stir-bar sorptive extraction-thermal desorption-gas chromatography-mass spectrometry analysis of pesticide residues in fruit-based soft drinks. Talanta 2011, 83, 1754–1762.
- Kashanian, S.; Dolatabadi, J.E.N. DNA binding studies of 2-tert-butylhydroquinone (TBHQ) food additive. Food Chem. 2009, 116, 743–747.
- Zurita, J.L.; Jos, A.; Peso, A.D.; Salguero, M.; López-Artíguez, M.; Repetto, G. Ecotoxicological effects of the antioxidant additive propyl gallate in five aquatic systems. Water Res. 2007, 41, 2599–2611.
- Iverson, F. In vivo studies on butylated hydroxyanisole. Food Chem. Toxicol. 1999, 37, 993–997.
- Botterweck, A.A.M.; Verhagen, H.; Goldbohm, R.A.; Kleinjans, J.; van den Brandt, P.A. Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: Results from analyses in the Netherlands Cohort Study. Food Chem. Toxicol. 2000, 38, 599–605.
- Singh, B.; Mense, S.M.; Remotti, F.; Liu, X.; Bhat, H.K. Antioxidant butylated hydroxyanisole inhibits estrogen-induced breast carcinogenesis in female ACI rats. J. Biochem. Mol. Toxicol. 2009, 23, 202–211.
- Zhang, Y.; Choksi, S.; Liu, Z.-G. Butylated hydroxyanisole blocks the occurrence of tumor-associated macrophages in tobacco smoke carcinogen-induced lung tumorigenesis. Cancers 2013, 5, 1643–1654.
- Liang, X.; Tang, Y.; Duan, L.; Cheng, S.; Luo, L.; Cao, X.; Tu, B. Adverse effect of sub-chronic exposure to benzo(a)pyrene and protective effect of butylated hydroxyanisole on learning and memory ability in male Sprague-Dawley rat. J. Toxicol. Sci. 2014, 39, 739–748.
- Simon, R.A. Adverse reactions to food additives. Curr. Allergy Asthma Rep. 2003, 3, 62–66.
- Horváthová, E.; Slameňová, D.; Bonatti, S.; Abbondandolo, A. Reduction of genotoxic effects of MNNG by butylated hydroxyanisole. Neoplasma 1999, 46, 356–362.
- Yang, X.; Song, W.; Liu, N.; Sun, Z.; Liu, R.; Liu, Q.S.; Zhou, Q.; Jiang, G. Synthetic phenolic antioxidants cause perturbation in steroidogenesis in vitro and in vivo. Environ. Sci. Technol. 2018, 52, 850–858.
- Dassarma, B.; Nandi, D.K.; Gangopadhyay, S.; Samanta, S. Hepatoprotective effect of food preservatives (butylated hydroxyanisole, butylated hydroxytoluene) on carbon tetrachloride-induced hepatotoxicity in rat. Toxicol. Rep. 2018, 5, 31–37.
- Pérez-Albaladejo, E.; Lacorte, S.; Porte, C. Differential toxicity of alkylphenols in JEG-3 human placental cells: Alteration of P450 aromatase and cell lipid composition. Toxicol. Sci. 2019, 167, 336–346.
- Valentao, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Antioxidative properties of cardoon (Cynara cardunculus L.) infusion against superoxide radical, hydroxyl radical, and hypochlorous acid. J. Agric. Food Chem. 2002, 50, 4989–4993.
- Phenolic Antioxidant Market Research Report–Forecast to 2023. Market Research Future. Available online: https://www.marketresearchfuture.com/reports/phenolic-antioxidant-market-3937#answer1 (accessed on 13 December 2020).
