Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Analytical
The Annonaceae family is widely distributed in subtropical and tropical regions. Several species of this family are known for their pharmacological and beneficial properties to human health, mainly attributed to flavonoids.
- graviola tree
- fruit
- leaves
1. Introduction
The Annonaceae family is widely distributed in subtropical and tropical regions. This family comprises a great diversity [1], with 180 genera and more than 3000 species. It stands out as the most prominent family in the Magnoliales order. The presence of many primitive morphological features and the ability to survive mass extinctions have characterized Annonaceae species as “living fossils” [2].
The botanical characteristics of the Annonaceae family may differ from species to species, depending on the origin, climate, and topography. This diversity can range from trees to shrubs to perennial vines, with elongated cylindrical intracellular resin channels and a broad and well-developed septate pith in the stems. The aromatic flowers bloom before they are fully developed. They are axillary, singular or grouped, hermaphroditic, and regular in shape. The fruits are made up of bunches of berries and are widely consumed due to their high nutritional value [2][3].
Annonaceae are very famous due to their wide use in traditional medicine. Several pharmacological studies have been conducted related to the medicinal use of the Annonaceae family, such as its use against pain, arthritis, rheumatism, and neuralgia, and in weight loss [4], anthelmintic and malaria [5], and antiplatelet [6] treatments.
This family also stands out for its wide variety of chemical constituents belonging to various phytochemical groups. Studies report the presence of cyclopeptides [7], flavonoids [8][9], terpenoids [10][11], lignans [12][13], steroids [14], tannins [8], volatile oils, resins [12], many alkaloids [15][16], and acetogenins [17]. Commercially, the most important Annonaceae are the species of the genus Annona [18]. Among the species of this genus, we can highlight plants such as pinha, marolo, graviola [19], araticum [20], marolinho [21], and bananinha [22].
Araticunzeiro plants are heterogeneous in terms of stem diameter (10.4 cm), plant height (3.7 m), and production (average of six fruits per plant), with production being the most variable characteristic [23]. The tree produces fruits with acceptable sensory characteristics, as well as significant nutritional and functional potentials [24], and their fruit is the most studied part of this plant. In this vein, the Araticum (Annona crassiflora Mart.) (Figure 1) is a fruit-bearing species native to the Brazilian Cerrado biome. When ripe, the araticum fruit has a flattened circular shape, rigid epicarp, and brown coloration [25]. The fruit has an oval or rounded shape, weighing from 0.5 to 4.5 kg, and its seeds are dark brown with a flat oval shape [26]. The harvest of this fruit takes place between September and April [27][28] and provides a great nutritional richness and a wide diversity of phytochemicals, such as carotenoids, folate, flavonoids, ascorbic acid, and vitamins A and E [29][30]. The fruit has reduced total titratable acidity (0.51 g citric acid/100 g) and pH (4.64) and high levels of total soluble solids (13 Brix) and moisture (74.30%), allowing its use in desserts such as sweets, jams, and yogurts. The fruit pulp contains high levels of carbohydrates (18.65%), lipids (3.78%), and energy value (113.65 kcal/100 g), and relatively low amounts of proteins (1.27%) [24].

Figure 1. Araticum (Annona crassiflora Mart.). Source: Illustration by Ribeiro, L.V., 2022.
The fruit and parts of the tree (leaves, stem, and peel) are the subjects of numerous research works due to their content of potentially bioactive compounds [31]. The biological activities of infusions, decoctions, or extracts of A. muricata leaves are related to their content of bioactive compounds, and these leaves are considered a rich source of bioactive compounds with potential uses in the formulation of therapeutic drugs or nutraceutical foods [32]. The fruit of the gravioleira tree, graviola (Annona muricata) also known as soursop (Figure 2), is a native fruit of North and South America and is currently distributed in subtropical and tropical regions. Its fruits are large and green in color [33]. The fruiting period of graviola generally occurs throughout the year; however, depending on the altitude, there are more defined seasons [34]. This species has a high concentration of secondary metabolites, such as flavonoids, terpenoids, alkaloids, and some lactones, in addition to minerals such as potassium, calcium, sodium, iron, and magnesium [35]. According to Fuentes (2021), in a bibliographic survey, the nutritional composition of soursop ranges from 55.4 to 81.83 (kcal) for total energy, 80.48–83.2% for moisture content, 0.69–1.10% for protein, 0.20–0.97% for lipid content, 12.50–18.23% for soluble carbohydrates, and 4.83–5.76% for dietary fiber content [36].
Figure 2. Graviola (Annona muricata). Source: Illustration by Ribeiro, L.V., 2022.
The atemoya tree a hybrid plant resulting from the cross between cherimoya (A. cherimola Mill.) and pinha (A. squamosa L.) [37] maintains the good qualities of both plants. The atemoya fruit (Annona cherimola Mill. × Annona squamosa L.) (Figure 3) has a small number of seeds, better conservation after harvest, absence of cracks and resistance to weeds, and improved biological control [38]. When pollinated, its shape is heart-shaped, ovate, or conical. The surface of the atemoya is smooth or may present prominence, and the pulp of this fruit has a white color. Regarding its phytochemicals, reports in the literature place the greatest emphasis on the class of acetogenins [39]. This fruit has approximately 65.18% moisture, 0.55% lipids, 1.24% proteins, 2.04% ash, 31.99% carbohydrates, and 1.55% total fiber [38]. Its leaves and stems stand out for their total phenol and flavonoid levels, as well as their antioxidant and antimicrobial activities [40].

Figure 3. Atemoya (Annona cherimola Mill. × Annona squamosa L.). Source: Illustration by Ribeiro, L.V., 2022.
The pine tree (Annona squamosa) is characterized by its small size, pivoting roots, and elongated leaves. These features also have several ramifications. The height of this plant varies from three to six meters, its flowers are small and can be isolated or in the form of a bunch, and its fruits are flat, ovoid, or heart-shaped [41]. The Annona squamosa L. fruit (Figure 4), popularly known as “ata”, “pinha”, or “fruta-do-conde”, is widely used for fresh consumption [42]. The fruit is widely known and consumed according to its medicinal and nutritional properties and its pleasant taste. It stands out for the presence of health-related components such as vitamins (A, B, C, E, and K1), antioxidants, polyunsaturated fatty acids, and the presence of essential minerals. In addition, this plant has a variety of compounds responsible for various activities such as the inhibition of insect attack, antimalarial, cytotoxic, immunosuppressive, and anti-HIV properties, and antiplatelet activity [43]. This fruit has an average caloric content of 95.3 (kcal), 70% moisture, 1.23% proteins, 0.21% lipids, 22.8% soluble carbohydrates, and 1.02% dietary fiber [44].

Figure 4. Pinha (Annona squamosa L.). Source: Illustration by Ribeiro, L.V., 2022.
Annona leptopetala is considered an endemic Brazilian tree. It is a woody, deciduous species with a high representation. This species’ reproductive and vegetative development is associated with the availability of water in nature [45][46]. The fruit (Figure 5), popularly known as “bananinha” [47], is the second most abundant species in the Brazilian Cerrado [46]. This species is unique in the genus, which has red flowers. From 2 to 9 m in height, its tree has elliptical to ovate leaves, with yellow, orange, or red apocarpous fruits, its flowering and fruiting ranges from November to April [47]. Bananinha is indicated in traditional medicine for the treatment of inflammation and tumors [48], and for its larvicidal potential [49], as well as its modulating effect on bacterial resistance to norfloxacin, in addition to a considerable antitumor effect in vivo [50]. The phytochemical study points to the presence of aporphine and tetrahydro-protoberberine alkaloids, in addition to acetogenins and a xanthone [51].

Figure 5. Bananinha (Annona leptopetala). Source: Illustration by Ribeiro, L.V., 2022.
Annona coriacea is considered an evergreen shrub or small tree [21]. Popularly known as “marolinho” and “cabeça-de-negro”, the species Annona coriacea occurs in some states of northeastern Brazil in an area of caatinga [52]. The height of this tree species varies from 3 to 18 m, with simple, obovate leaves, glabrous on the ventral surface, the base is often chordate and the margin wavy; the flowers are solitary, terminal, and bushy, with fleshy petals ranging in color from orange to orange-pink [21][53]. The fruit (Figure 6) is globular or elongated, white pulp, watery, soft, smooth rind, or fleshy scales. In Brazil, it is popularly used against chronic diarrhea, malaria, helminths, and leishmaniasis [53]. Many biological activities are described for A. coriacea, including insecticidal activity [50][54], antiprotozoal [55], phytotoxic [56], cytoprotective [57], antiproliferative, cytotoxic, and enzymatic inhibitory effects [58]. Phytochemical studies report the presence of polyphenols [57], alkaloids [59], and volatile compounds [60].

Figure 6. Marolinho (Annona coriácea). Source: Illustration by Ribeiro, L.V., 2022.
Several reports in the literature show that such varieties are correlated with antitumor, antioxidant, and antiparasitic activities, as well as antimicrobial and anti-inflammatory effects [24][60][61]. Anaya-Esparza and collaborators (2020) show in a bibliographical review that Annona species are widely used in folk medicine, demonstrating great importance. Their traditional use is associated with treating fever, diarrhea, dysentery, hematuria, urethritis, asthma, and parasitic and hepatic diseases, in addition to exerting antispasmodic, laxative, antiemetic, antisudorific, antitussive, antiflu, and antidepressant effects [62]. Several of these species are well known due to their pharmacological properties and benefits to human health, mainly attributed to the presence of flavonoids, the main bioactive constituent found within them [18].
Phenolic compounds belong to a large compound class that includes both simple and complex structures with at least one aromatic ring. We can highlight several derived classes of phenolic compounds and their basic skeleton: C6 simple phenols, benzoquinones; C6-C1 phenolic acids; C6-C2 acetophenones and phenylacetic acids; C6-C3 phenylpropanoids; C6-C3 coumarins; C6-C4 naphthoquinones; C6-C1-C6 xanthones; C6-C2-C6 stilbenes and anthraquinones; C6-C3-C6 flavonoids and isoflavonoids; (C6-C3)2 lignans; (C6-C3-C6)2 diflavonoids; (C6)n plant melanins; (C6-C3)n lignins; (C6-C1)n atemoya tannins; (C6-C3-C6)n condensed tannins. Figure 7 shows the simplified biosynthetic route of phenolic compounds, highlighting flavonoids.
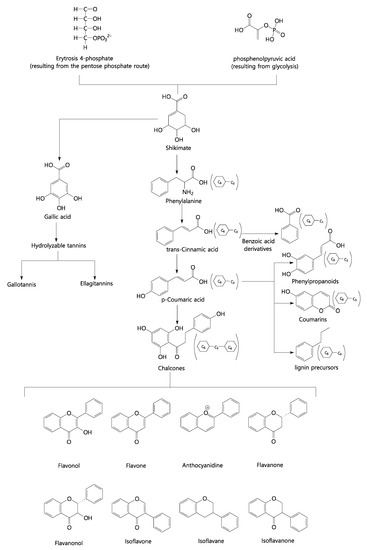
Figure 7. Simplified biosynthetic pathway of phenolic compounds. Source: Authors, 2022.
Flavonoids are part of the most relevant and diversified group of phenolic compounds among natural products [63] and are widely distributed in the plant kingdom [64][65]. They stand out for having great economic attractiveness due to their various properties beneficial to health [66]. In the Annona genus, the most common flavonoid classes are quercetin-3-O-rhamnoside, luteolin-7-O-glucoside, kaempferol-3-O-galactoside, and their derivatives [67]. Esses compostos pertencem a uma importante classe de metabólitos secundários [ 68 ] , além de possuírem a virtude de sua ampla variedade de atividades biológicas e terapêuticas, demonstradas tanto in vitro quanto in vivo [ 65 ] . Podemos assim destacar a correlação destes compostos com a capacidade antioxidante, propriedades anti‐inflamatórias, antitumorais, antivirais, entre outras [ 60 ] [ 69 ] .
This entry is adapted from the peer-reviewed paper 10.3390/plants11212855
References
- Barosa, L.T.C.; Vega, M.R.G. Diterpenos Do Gênero Xylopia. Rev. Virtual Quim. 2017, 9, 1712–1733.
- Attiq, A.; Jalil, J.; Husain, K. Annonaceae: Breaking the Wall of Inflammation. Front. Pharmacol. 2017, 8, 752.
- Hamonnière, M.; Leboeuf, M.; Cavé, A. Alcaloides Aporphiniques et Composés Terpéniques Du Polyalthia Oliveri. Phytochemistry 1977, 16, 1029–1034.
- Cercato, L.M.; White, P.A.S.; Nampo, F.K.; Santos, M.R.V.; Camargo, E.A. A Systematic Review of Medicinal Plants Used for Weight Loss in Brazil: Is There Potential for Obesity Treatment? J. Ethnopharmacol. 2015, 176, 286–296.
- Bhalk, R.D.; Chavan, M.J. Analgesic and CNS Depressant Activities of Extracts of Annona Reticulata Linn. Bark. Phytopharmacology 2011, 1, 160–165.
- González-Esquinca, A.R.; De-La-Cruz-Chacón, I.; Castro-Moreno, M.; Orozco-Castillo, J.A.; Riley- Saldaña, C.A. Alkaloids and Acetogenins in Annonaceae Development: Biological Considerations. Rev. Bras. Frutic. 2014, 36, 1–16.
- Moghadamtousi, S.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.; Kadir, H. Annona Muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int. J. Mol. Sci. 2015, 16, 15625–15658.
- Brito, H.O.; Noronha, E.P.; França, L.M.; Brito, L.M.O.; Prado, M.S.-A. Análise Da Composição Fitoquímica Do Extrato Etanólico Das Folhas Da Annona Squamosa (ATA). Rev. Bras. Farm. 2008, 89, 180–184.
- Chokchaisiri, R.; Chaichompoo, W.; Chalermglin, R.; Suksamrarn, A. Potent Antiplasmodial Alkaloids and Flavonoids from Dasymaschalon Acuminatum. Rec. Nat. Prod. 2015, 9, 243–246.
- Chang, F.-R.; Yang, P.-Y.; Lin, J.-Y.; Lee, K.-H.; Wu, Y.-C. Bioactive Kaurane Diterpenoids from Annona Glabra. J. Nat. Prod. 1998, 61, 437–439.
- Rabelo, S.V.; Quintans, J.d.S.S.; Costa, E.V.; Almeida, J.R.G.d.S.; Júnior, L.J.Q. Annona Species (Annonaceae) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 221–229.
- Ferreira, L.; Perestrelo, R.; Camara, J. Comparative Analysis of the Volatile Fraction from Annona Cherimola Mill. Cultivars by Solid-Phase Microextraction and Gas Chromatography–Quadrupole Mass Spectrometry Detection. Talanta 2009, 77, 1087–1096.
- Rayanil, K.; Sutassanawichanna, W.; Suntornwat, O.; Tuntiwachwuttikul, P. A New Dihydrobenzofuran Lignan and Potential α -Glucosidase Inhibitory Activity of Isolated Compounds from Mitrephora Teysmannii. Nat. Prod. Res. 2016, 30, 2675–2681.
- Moreira, I.C.; Lago, J.H.G.; Roque, N.F. Alkaloid, Flavonoids and Terpenoids from Leaves and Fruits of Xylopia Emarginata (Annonaceae). Biochem. Syst. Ecol. 2003, 31, 535–537.
- da Silva, D.B.; Matos, M.d.F.C.; Nakashita, S.T.; Misu, C.K.; Yoshida, N.C.; Carollo, C.A.; Fabri, J.R.; Miglio, H.d.S.; Siqueira, J.M. de Isolamento e Avaliação Da Atividade Citotóxica de Alguns Alcalóides Oxaporfínicos Obtidos de Annonaceae. Quim. Nova 2007, 30, 1809–1812.
- Soares, E.R.; da Silva, F.M.A.; de Almeida, R.A.; de Lima, B.R.; Filho, F.A.d.S.; Barison, A.; Koolen, H.H.F.; Pinheiro, M.L.B.; de Souza, A.D.L. Direct Infusion ESI-IT-MSn Alkaloid Profile and Isolation of Tetrahydroharman and Other Alkaloids from Bocageopsis Pleiosperma Maas (Annonaceae). Phytochem. Anal. 2015, 26, 339–345.
- Alali, F.Q.; Liu, X.-X.; McLaughlin, J.L. Annonaceous Acetogenins: Recent Progress. J. Nat. Prod. 1999, 62, 504–540.
- Bermejo, A.; Figadère, B.; Zafra-Polo, M.-C.; Barrachina, I.; Estornell, E.; Cortes, D. Acetogenins from Annonaceae: Recent Progress in Isolation, Synthesis and Mechanisms of Action. Nat. Prod. Rep. 2005, 22, 269–303.
- Krinski, D.; Massaroli, A.; Machado, M. Potencial Inseticida de Plantas Da Família Annonaceae. Rev. Bras. Frutic. 2014, 36, 225–242.
- De Melo, A.P.C.; Seleguini, A.; Leite, A.F.; De Souza, E.R.B.; Naves, R.V. Fenologia Reprodutiva Do Araticum e Suas Implicações No Potencial Produtivo. Comun. Sci. 2015, 6, 495.
- Rocha, G.N.d.S.A.O.; Dutra, L.M.; Lorenzo, V.P.; Almeida, J.R.G.d.S. Phytochemicals and Biological Properties of Annona Coriacea Mart. (Annonaceae): A Systematic Review from 1971 to 2020. Chem. Biol. Interact. 2021, 336, 109390.
- Brito, M.T.; Ferreira, R.C.; Beltrão, D.M.; Moura, A.P.G.; Xavier, A.L.; Pita, J.C.L.R.; Batista, T.M.; Longato, G.B.; Ruiz, A.L.T.G.; de Carvalho, J.E.; et al. Antitumor Activity and Toxicity of Volatile Oil from the Leaves of Annona Leptopetala. Rev. Bras. Farmacogn. 2018, 28, 602–609.
- Pimenta, A.C.; Silva, P.S.d.R.; Zuffellato-Ribas, K.C.; Koehler, H.S. Caracterização de Plantas e de Frutos de Araticunzeiro (Annona Crassiflora Mart.) Nativos No Cerrado Matogrossense. Rev. Bras. Frutic. 2014, 36, 892–899.
- Arruda, H.S.; Pastore, G.M. Araticum (Annona Crassiflora Mart.) as a Source of Nutrients and Bioactive Compounds for Food and Non-Food Purposes: A Comprehensive Review. Food Res. Int. 2019, 123, 450–480.
- Cardoso, L.; de Araticum, M.; Jatobá, C. Mangaba e Pequi Do Cerrado de Minas Gerais: Ocorrência e Conteúdo de Carotenóides e Vitaminas; Universidade Federal de Viçosa: Viçosa, Brazil, 2011.
- Arruda, H.S.; Pereira, G.A.; de Morais, D.R.; Eberlin, M.N.; Pastore, G.M. Determination of Free, Esterified, Glycosylated and Insoluble-Bound Phenolics Composition in the Edible Part of Araticum Fruit (Annona Crassiflora Mart.) and Its by-Products by HPLC-ESI-MS/MS. Food Chem. 2018, 245, 738–749.
- Arruda, H.S.; Fernandes, R.V.d.B.; Botrel, D.A.; Almeida, M.E.F. Frutos Do Cerrado: Conhecimento e Aceitação de Annona Crassiflora Mart. (Araticum) e Eugenia Dysenterica Mart. (Cagaita) Por Crianças Utilizando o Paladar e a Visão. J. Health Biol. Sci. 2015, 3, 224.
- de Morais, E.C.; Patias, S.G.d.O.; Ferreira, N.S.d.S.; Picanço, N.F.M.; Rodrigues, E.C.; Nascimento, E.; Faria, R.A.P.G. e Compostos Bioativos e Características Físico-Químicas de Polpa de Araticum in Natura e Pasteurizada. Braz. J. Food Technol. 2017, 20, e2016142.
- Cardoso, L.d.M.; Oliveira, D.d.S.; Bedetti, S.d.F.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Araticum ( Annona Crassiflora Mart.) from the Brazilian Cerrado: Chemical Composition and Bioactive Compounds. Fruits 2013, 68, 121–134.
- Reis, A.F.; Schmiele, M. Características e Potencialidades Dos Frutos Do Cerrado Na Indústria de Alimentos. Braz. J. Food Technol. 2019, 22, e2017150.
- Lee, C.H.; Lee, T.H.; Ong, P.Y.; Wong, S.L.; Hamdan, N.; Elgharbawy, A.A.M.; Azmi, N.A. Integrated Ultrasound-Mechanical Stirrer Technique for Extraction of Total Alkaloid Content from Annona Muricata. Process Biochem. 2021, 109, 104–116.
- Nolasco-González, Y.; Chacón-López, M.A.; Ortiz-Basurto, R.I.; Aguilera-Aguirre, S.; González-Aguilar, G.A.; Rodríguez-Aguayo, C.; Navarro-Cortez, M.C.; García-Galindo, H.S.; García-Magaña, M.d.L.; Meza-Espinoza, L.; et al. Annona Muricata Leaves as a Source of Bioactive Compounds: Extraction and Quantification Using Ultrasound. Horticulturae 2022, 8, 560.
- Mishra, S.; Ahmad, S.; Kumar, N.; Sharma, B.K. Annona Muricata (the Cancer Killer): A Review. Glob. J. Pharm. Res. 2013, 2, 1613–1618.
- Pinto, A.C.d.Q.; Cordeiro, M.C.R.; de Andrade, S.R.M.; Ferreira, F.R.; Filgueiras, H.A.d.C.; Alves, R.E.; Kinpara, D.I. Annona Species, 1st ed.; Williams, J.T., Smith, R.W., Hughes, A., Haq, N., Clement, C.R., Eds.; International Centre for Underutilised Crops, University of Southampton: Southampton, UK, 2005; ISBN 0854327851.
- Chen, A.M.; Farwell, D.G.; Luu, Q.; Vazquez, E.G.; Lau, D.H.; Purdy, J.A. Intensity-Modulated Radiotherapy Is Associated with Improved Global Quality of Life among Long-Term Survivors of Head-and-Neck Cancer. Int. J. Radiat. Oncol. 2012, 84, 170–175.
- Fuentes, L.M.H.; González, E.M.; Magaña, M.d.L.G.; Esparza, L.M.A.; González, Y.N.; Villagrán, Z.; Torres, S.G.; Monreal, J.J.V.; Flores, D.A.M. Current Situation and Perspectives of Fruit Annonaceae in Mexico: Biological and Agronomic Importance and Bioactive Properties. Plants 2021, 11, 7.
- de Oliveira, M.C.; Ferreira, G.; Guimarães, V.F.; Dias, G.B. Germinação de Sementes de Atemoia (Annona Cherimola Mill. × A. Squamosa L.) Cv “Gefner” Submetidas a Tratamentos Com Ácido Giberélico (GA3) e Ethephon. Rev. Bras. Frutic. 2010, 32, 544–554.
- dos Santos, W.N.L.; Sauthier, M.C.S.; Cavalcante, D.D.; Benevides, C.M.J.; Dias, F.S.; Santos, D.C.M.B. Mineral Composition, Nutritional Properties, Total Phenolics and Flavonoids Compounds of the Atemoya Fruit (Annona Squamosa L. × Annona Cherimola Mill.) and Evaluation Using Multivariate Analysis Techniques. An. Acad. Bras. Cienc. 2016, 88, 1243–1252.
- Rabêlo, S.V. Revisão de Alcaloides Do Gênero Annona, Estudo Fitoquímico e Avaliação Da atividade Biológica de Atemoia (Annona Cherimolax Annona Squamosa); Universidade Federal do Vale do São Francisco: Petrolina, Brazil, 2014.
- Rabêlo, S.V.; Costa, E.V.; Barison, A.; Dutra, L.M.; Nunes, X.P.; Tomaz, J.C.; Oliveira, G.G.; Lopes, N.P.; Santos, M.d.F.C.; Almeida, J.R.G.d.S. Alkaloids Isolated from the Leaves of Atemoya (Annona Cherimola × Annona Squamosa). Rev. Bras. Farmacogn. 2015, 25, 419–421.
- Fortuño, J.N. Guía de Las Frutas Cultivadas: Identificación y Cultivo, 1st ed.; Ediciones Mundi-Prensa: Madrid, Spain, 2001; ISBN 848476009X.
- Bataglion, G.A.; da Silva, F.M.A.; Eberlin, M.N.; Koolen, H.H.F. Determination of the Phenolic Composition from Brazilian Tropical Fruits by UHPLC–MS/MS. Food Chem. 2015, 180, 280–287.
- Liu, K.; Li, H.; Yuan, C.; Huang, Y.; Chen, Y.; Liu, J. Identification of Phenological Growth Stages of Sugar Apple (Annona Squamosa L.) Using the Extended BBCH-Scale. Sci. Hortic. 2015, 181, 76–80.
- CNFlora Annona Squamosa. Available online: http://cncflora.jbrj.gov.br/portal/pt-br/profile/Annona squamosa (accessed on 19 August 2022).
- de Lima, A.L.A.; Sampaio, E.V.d.S.B.; de Castro, C.C.; Rodal, M.J.N.; Antonino, A.C.D.; de Melo, A.L. Do the Phenology and Functional Stem Attributes of Woody Species Allow for the Identification of Functional Groups in the Semiarid Region of Brazil? Trees 2012, 26, 1605–1616.
- Agra, M.d.F.; de Freitas, P.F.; Barbosa-Filho, J.M. Synopsis of the Plants Known as Medicinal and Poisonous in Northeast of Brazil. Rev. Bras. Farmacogn. 2007, 17, 114–140.
- Júnior, J.T.C.; de Moura, J.C.; Lisboa, M.A.N.; Cruz, G.V.; Gonçalves, B.L.M.; Barreto, E.S.d.S.T.; Barros, L.M.; Drumond, M.A.; Mendonça, A.C.A.M.; Rocha, L.S.G.; et al. Phytosociology, Diversity and Floristic Similarity of a Cerrado Fragment on Southern Ceará State, Brazilian Semiarid. Sci. For. 2021, 49.
- Feitosa, E.M.A.; Arriaga, Â.M.C.; Santiago, G.M.P.; de Lemos, T.L.G.; de Oliveira, M.C.F.; Vasconcelos, J.N.; Lima, J.Q.; Malcher, G.T.; do Nascimento, R.F.; Braz-Filho, R. Chemical Composition and Larvicidal Activity of Rollinia Leptopetala (Annonaceae). J. Braz. Chem. Soc. 2009, 20, 375–378.
- Costa, V.C.d.O.; Tavares, J.F.; Queiroga, C.S.; Castello-Branco, M.V.S.; Diniz, M.F.F.M.; de Lima, C.U.G.B.; Santos, B.V.d.O.; Pita, J.C.L.R.; da Silva, M.S.; Sette, I.M.F. Constituintes Químicos Das Folhas de Rollinia Leptopetala R. E. Fries. Quim. Nova 2012, 35, 138–142.
- Arriaga, Â.M.C.; Feitosa, E.M.A.; Lemos, T.L.G.; Santiago, G.M.P.; Lima, J.Q.; De Oliveira, M.C.F.; Vasconcelos, J.N.; Rodrigues, F.E.A.; Gomes, T.B.M.; Braz-Filho, R. Chemical Constituents and Insecticidal Activity of Rollinia Leptopetala (Annonaceae). Nat. Prod. Commun. 2008, 3, 1934578X0800301.
- CNFlora Annona Leptopetala. Available online: http://www.cncflora.jbrj.gov.br/portal/pt-br/profile/Annona leptopetala (accessed on 22 January 2022).
- Benites, R.; Formagio, A.; Argandoña, E.; Volobuff, C.; Trevizan, L.; Vieira, M.; Silva, M. Contents of Constituents and Antioxidant Activity of Seed and Pulp Extracts of Annona Coriacea and Annona Sylvatica. Braz. J. Biol. 2015, 75, 685–691.
- Freitas, A.F.; Pereira, F.F.; Formagio, A.S.N.; Lucchetta, J.T.; Vieira, M.C.; Mussury, R.M. Effects of Methanolic Extracts of Annona Species on the Development and Reproduction of Spodoptera Frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 2014, 43, 446–452.
- de Toledo, C.E.M.; Britta, E.A.; Ceole, L.F.; Silva, E.R.; de Mello, J.C.P.; Dias Filho, B.P.; Nakamura, C.V.; Ueda-Nakamura, T. Antimicrobial and Cytotoxic Activities of Medicinal Plants of the Brazilian Cerrado, Using Brazilian Cachaça as Extractor Liquid. J. Ethnopharmacol. 2011, 133, 420–425.
- Novaes, P.; Torres, P.B.; dos Santos, D.Y.A.C. Biological Activities of Annonaceae Species Extracts from Cerrado. Braz. J. Bot. 2016, 39, 131–137.
- Junior, J.A.; Coutinho, H.; Boris, T.; Cristo, J.; Pereira, N.; Figueiredo, F.; Cunha, F.; Aquino, P.; Nascimento, P.; Mesquita, F.; et al. Chemical Characterization and Cytoprotective Effect of the Hydroethanol Extract from Annona Coriacea Mart. (Araticum). Pharmacognosy Res. 2016, 8, 253.
- Formagio, A.S.N.; Vieira, M.C.; Volobuff, C.R.F.; Silva, M.S.; Matos, A.I.; Cardoso, C.A.L.; Foglio, M.A.; Carvalho, J.E. In Vitro Biological Screening of the Anticholinesterase and Antiproliferative Activities of Medicinal Plants Belonging to Annonaceae. Braz. J. Med. Biol. Res. 2015, 48, 308–315.
- Machado, A.R.T.; Lage, G.A.; Medeiros, F.d.S.; Filho, J.D.d.S.; Pimenta, L.P.S. Quantitative Analysis of Trigonelline in Some Annona Species by Proton NMR Spectroscopy. Nat. Products Bioprospect. 2013, 3, 158–160.
- Siqueira, C.A.T.; Oliani, J.; Sartoratto, A.; Queiroga, C.L.; Moreno, P.R.H.; Reimão, J.Q.; Tempone, A.G.; Fischer, D.C.H. Chemical Constituents of the Volatile Oil from Leaves of Annona Coriacea and in Vitro Antiprotozoal Activity. Rev. Bras. Farmacogn. 2011, 21.
- Flambó, D.F.A.L.P. Atividades Biológicas Dos Flavonoides: Atividade Antimicrobiana; Universidade Fernando Pessoa—Faculdade de Ciências da Saúde: Porto, Portugal, 2013.
- Mannino, G.; Gentile, C.; Porcu, A.; Agliassa, C.; Caradonna, F.; Bertea, C.M. Chemical Profile and Biological Activity of Cherimoya (Annona Cherimola Mill.) and Atemoya (Annona Atemoya) Leaves. Molecules 2020, 25, 2612.
- Anaya-Esparza, L.M.; García-Magaña, M.d.L.; Domínguez-Ávila, J.A.; Yahia, E.M.; Salazar-López, N.J.; González-Aguilar, G.A.; Montalvo-González, E. Annonas: Underutilized Species as a Potential Source of Bioactive Compounds. Food Res. Int. 2020, 138, 109775.
- Simões, C.M.O.; Schenkel, E.P.; de Mello, J.C.P.; Mentz, L.A.; Petrovick, P.R. Farmacognosia: Do Produto Natural Ao Medicamento, 1st ed.; Artmed Guelph: Guelph, ON, Canada, 2016.
- da Silva, L.R.; Martins, L.d.V.; Calou, I.B.F.; de Deus, M.d.S.M.; Ferreira, P.M.P.; Peron, A.P. Flavonóides: Constituição Química, Ações Medicinais e Potencial Tóxico. Acta Toxicológica Argent. 2015, 23, 36–43.
- de Almeida, A.S.; Santos, A.F. dos Flavonoides Do Gênero Annona. Divers. J. 2018, 3, 475.
- Machado, H.; Nagem, T.J.; Peters, V.M.; Fonseca, C.S.; Oliveira, T.T. de Flavonóides e Seu Potencial Terapêutico. Bol. Cent. Biol. Reprodução 2008, 27, 33–39.
- Santos, D.Y.A.C.; Salatino, M.L.F. Foliar Flavonoids of Annonaceae from Brazil: Taxonomic Significance. Phytochemistry 2000, 55, 567–573.
- Cristina Marcucci, M.; Salatino, A.; Farias Azevedo de Magalhães Oliveira, L.; Passarelli Gonçalves, C.; Accessible Methodologies for Quantification of Flavonoids and Total Phenols in Propolis.. Rev. Virtual Química 2021, 13, 61-73, 10.21577/1984‐6835.20200131.
- Ramos, A.L.C.C.; Minighin, E.C.; Soares, I.I.C.; Ferreira, R.M. de S.B.; Sousa, I.M.N. de; Augusti, R.; Labanca, R.A.; Araújo, R.L.B. de; Melo, J.O.F.; Evaluation of the total phenolic content, antioxidative capacity, and chemical fingerprint of Annona crassiflora Mart. Bioaccessible molecules. Food Res. Int. 2023, 165, 112514, 10.1016/j.foodres.2023.112514.
This entry is offline, you can click here to edit this entry!
