Porous silica nanosphere is one of the conventional and common form of silica nanomaterials in the field of nanotechnology. Most common nanopsheres of porous silica are MCM-41, SBA-15 etc. Wide range of applications say catalytic, biological, energy applications have been investigated and reported using this materials.
- Spherical nanoparticles
- porous structure
1. Introduction
Among all of the morphologies, reports on spherical type silica and organosilica nanoparticles with the nanoscale level porosity are most abundant in literature. Silica particles can be found with pores the range of micropore (<2 nm) to mesopore (2–50 nm) arranged in an ordered and non-ordered way[1].
2. Synthesis Pathway
In 1956, Kolbe et al., first synthesized highly dispersed non-porous silica particles with the help of silicon alkoxide hydrolysis and condensation principle in alcohol[2]. Later, the controlled synthesis of micrometer-sized monodispersed silica sphere has been mentioned by Stöber et al., in 1968[3]. They used ammonia as base as well as morphology-controlling catalyst to produce spherical particles by the hydrolysis of alkyl silicate in alcoholic medium. For surfactant-free synthesis of mesoporous silica nanosphere with tunable pore diameter (100–230 nm) role of ammonia has been proved to be very crucial. Here, a facile two step pathway by premixing three components TEOS-H2O-EtOH with definite molar ratios followed by addition of ammonia, then the treatment of the resulting silica by water dilution and soft-etching with ammonia produce monodispersed porous silica sphere[4]. Usually, mesostructured spherical silica material, like MCM-41 with hexagonally ordered mesopores, can be produced based on the condensation of self-assembled cationic surfactant and hydrolysed silica precursor, tetraethyl orthosilicate or TEOS under highly alkaline condition[5]. Additionally, instead of TEOS, use of commercially available silica gel say, Lichrosphere 100 results pseudomorphic synthesis of MCM-41 with perfect spherical shape[6]. In the same way, pseudomorphic synthesis of large size spherical shaped Co-doped MCM-41 has been reported by Lim et al., who synthesized the material using commercial silica, cobalt salt and CTAB surfactant in highly basic medium[7]. Presence of Na ion in the reaction medium inhibits the homogeneous distribution of Co species in the silica matrices. Thus, well-defined spherical morphology of Co-MCM-41 was achieved by optimizing the autoclaving time to four days. On the other hand, in HCl acidic media long ageing time has also been employed by Alonso’s group to synthesize functionalized silica sphere using CTAB, TEOS, and isopropanol based on spray-drying method[8].
Sometimes, by using weakly basic amino acid like arginine the hydrolysis of TEOS can be controlled at moderate pH (9–10), which results in the formation of discrete silica nanosphere of 20–40 nm size[9]. Moreover, the size of the particles can be varied significantly by changing the stirring rate, amount of water, or cationic surfactant C16TMACl. Surfactant cetyltrimethylammonium chloride (C16TMACl) has also been used by Qiao et al., in order to synthesize uniform mesoporous spherical silica sphere at moderate pH = 6–10 in the presence of certain suitable additives like diethanol amine, triethanol amine, ammonia or any inorganic salts[10]. Monodispersed silica nanospheres can also be obtained simply using sodium acetate additive in the presence of C16TMACl surfactant and TEOS at moderate reaction temperature ca. 60 °C without adding any other alkalis[11]. In order to control the particle growth in nanometre range, the use of amino acid like lysine has been mentioned by Nandiyanto and his group. Here, the synthesis of mesoporous spherical silica sphere of 20–80 nm diameter was done using styrene and CTAB surfactant in water-octane media[12]. Styrene is polymerized in the reaction medium in order to produce polystyrene that acts as a template for this silica preparation, because a comparative study showed that, with increasing styrene concentration, particle size and pore size both gradually increase. Figure 1 shows a schematic representation for synthesis of mesoporous silica nanoparticles in the presence of CTAB-PS and amino acid lysine.
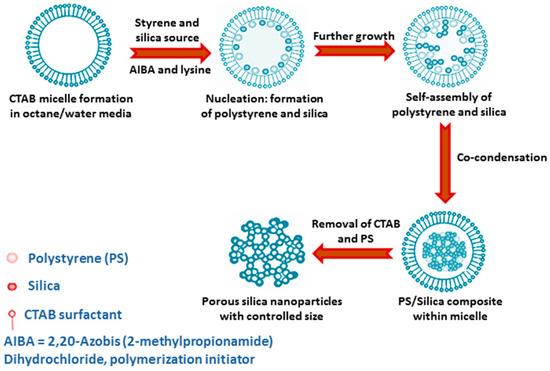
Figure 1. A schematic representation for mesoporous silica nanoparticles formation in presence of Cetyltrimethylammonium bromide-Polystyrene (CTAB-PS) and amino acid lysine.
In 2010, Polshettiwar et al., mentioned the formation of fibrous silica nanospheres while using cationic template like cetylpyridinium bromide (CPB) or CTAB and urea as hydrolysing agent in a solvent mixture of cylcohexane, pentanol, and water[13]. The role of non-polar template chain cetyl group and the polar head pyridinium or trimethylammonium group to produce this well-defined fibrous morphology is very crucial, owing to the surfactant packing parameter. Not only that, fabrication of this fibrous nanosilica sphere is highly dependent on precise control of urea amount as well as proper selection of combined solvent, cyclohexane, and pentanol. It was also observed that the resulting silica spheres have very high mechanical and thermal stability.
While using a neutral template like octylamine or dodecylamine, uniform monodispersed spherical silica and thiol (SH-)-functionalized silica can be obtained in highly acidic solution by using a water-ethanol solvent mixture, as revealed by Kosuge et al.[14]. Additionally, when compared to octylamine, dodecylamine has been proved to be more effective structure-directing agent to produce ordered mesoporous silica and organosilica nanospheres with higher morphological quality. In HCl medium, Coulombic interaction between charged species, say long-chain protonated amine (S+), coordinating anions of Cl- (X−), and positively charged silicate oligomer (I+), facilitates the S+X-I+ type mechanism, which favours the formation of this silica and functionalized silica hard spheres. Similar mechanism was observed in case of hard sphere Al-doped porous silica synthesis[15]. Another organosilica, say rhodamine spirolacturm derivative functionalized mesoporous silica synthesized using CTAB-NaOH mixture in aqueous media, has also produced uniform spherical type particles of 75–85 nm dimension[16]. Spherical mesoporous silica particles with diameter of 65–740 nm have been prepared by Nooney et al., by using a varying quantity of the initial reagents and changing template from cationic to neutral or reaction media from homogeneous to heterogeneous[17].
The formation of monodispersed silica nanosphere while using Pluronic triblock copolymer templates and sodium silicate in strong acidic aqueous media is highly dependent on the parameter like presence of counterions viz. Cl− and NO3−, which control the hydrophilicity of the reaction media resulting precise spherical shape[18]. Additionally, the particle size has been monitored by changing the reactants ratio, stirring speed, or by mixing of surfactants. Pluronic template F127 can also act as particle dispersion agent to synthesize monodispersed cubic mesostructured MCM-48 silica nanosphere in a CTAB templated basic water-ethanol media[19]. A diluted solution of CTAB and higher concentration of F127 facilitates the formation of well-defined smaller sized MCM-48 nanoparticles. In the similar way, a combination of surfactants CTAB and Brij-56 has been employed by He et al., in order to synthesize highly dispersed ordered mesoporous spherical silica nanoparticles under buffer maintained neutral pH condition[20]. Here also, the role of non-ionic surfactant Brij-56 is to improve the morphology and dispersivity of the silica nanoparticles, although the mesostructure was little disrupted by this. Monodispersed silica microsphere can be obtained by the evaporation induced CTAB-Brij 58 templating method while using vibrating orifice aerosol generator, as reported by Rama Rao et al.[21].
Use of a dispersing agent to synthesize well-dispersed three-dimensional (3D) porous silica sphere has also been reported by Fu and his co-workers [22]. They used carboxymethyl cellulose as dispersing agent in a dodecyltrimethylammonium bromide surfactant-mediated to fabricate cubic silica nanoparticles of ca. 60 nm via the rapid two-step pH-modulated method. Huo et al., reported the preparation of porous hard sphere silica particles using tetrabutyl orthosilicate (TBOS) as silica source in aqueous NaOH media[23]. Here, the desired morphology of silica is obtained while hydrophobic TBOS and butyl alcohol that was produced from the hydrolysis of TBOS in basic media creates oil-in-water emulsion and stabilized by the quaternary ammonium CTAB surfactant. Han et al. reported another strategy using mixed surfactants. They used triblock copolymer surfactant (e.g., F127, P123) and cationic fluorocarbon surfactant FC-4 for the preparation of well-dispersed mesoporous silica nanosphere in weakly acidic media[24]. The role of FC-4 is to get ultrafine particles by controlling the growth of particle, while mesostructure formation is facilitated by copolymer template.
Highly dispersed spherical shaped colloidal mesoporous silica (CMS) functionalized with different organic groups, like vinyl-, benzyl-, phenyl-, cyano-, mercapto-, aminopropyl-, etc., can be synthesized in a combined media of triethanolamine (TEA) and C16TMACl using mixture of TEOS and a variety of organoalkoxysilanes[25]. In the same way, colloidal silica nanoparticles with spherical morphology have also been reported by Yamada et al., who showed that by using different alkoxysilane (Si(OR)4, R = CH3, C2H5, C3H7, C4H9), four types of mesoporous silica nanospheres with different diameters (20–80 nm) can be prepared due to different hydrolysis rate of the silica precursors[26]. Another monodispersed colloidal suspension of porous silica spheres of very small dimension (15–30 nm) have been synthesized while using CTAB surfactant, triethanolamine catalyst, silica precursors TEOS, and phenyl functionalized silica, followed by extraction using HCl-ethanol and ammonium nitrate-ethanol[27]. A homogeneous thin film was also successfully prepared using this small nanoparticle suspension. In 2016, Curcumin based fluorescent organic-inorganic hybrid colloidal PMO is prepared by Datz et al., with the help of a mixture of Curcumin functionalized silica and bis(triethoxysilyl)ethane (BTEE) without TEOS in CTAB mediated alkaline water-ethanol media[28].
Zhang et al. reported the large scale production of well-dispersed porous silica sphere[29]. They showed that the fabrication of nanospherical silica with <130 nm particle size can be done by while using an appropriate cationic surfactant, cetyltrimethylammonium (CTA+) with suitable counterions (Tosylate, Tos−, or bromide, Br−) in the presence of small organic amines (SOAs), including triethyleneamine, triethanolamine, and 2-amino-2-(hydroxymethyl)propane-1,3-diol as minerali- zing agents. Meso-channelled spherical morphology, including stellate, raspberry, and worm-like structure, is controlled by adjusting proper SOA concentration and counterion types. The method results in the development of ‘weak templating condition’ (low silanolate density at pH = 7 due to high concentration of Tos− around CTA+ micelle) and ‘strong templating condition’ (high SOA concentration or presence of Br- ions and high silanolate density), which also enables high yield and large-scale production of mesoporous silica nanospheres. Figure 2 elaborates the formation pathways of silica-template organic-inorganic composite nanostructures. Yu et al. prepared uniform spherical silica nanospheres with dendritic pore channels, using imidazolium ionic liquids (ILs) with different alkyl lengths as cosurfactants in presence of triethanolamine, cetyltrimethylammonium tosylate, and TEOS[30].
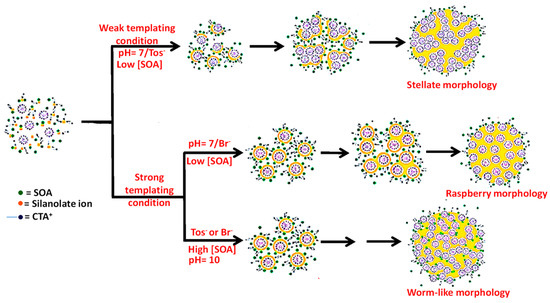
Figure 2. Schematic representations of pathways of silica-template organic-inorganic composite nanostructures formation under various conditions.
Surface functionalization of silica materials has been demonstrated by Bagwe et al. in order to reduce nanoparticle aggregation[31]. Surface modification was carried out using TEOS and different organosilanes in aqueous ammonia media based on a water-in-oil (W/O) or reverse microemulsion method. It was observed that the surface modification of silica with amine groups is further supported by an inert group (e.g., methyl phosphonate), for which highly monodispersed silica nanospheres have been obtained. Cheng et al. also reported well-dispersed mesoporous silica nanoparticles by proper functionalization[32].
Modified Stöber method has been employed for synthesis of well-defined spherical and monodispersed PMO by Rebbin and co-workers[33]. They used different long-chain tetraalkylammonium halide as surfactants and 1,2-bis(trimethoxysilyl)ethane (BTME) as silica precursors in ammoniacal water-ethanol media in order to obtain this PMO. Using the similar method, another PMO nanosphere having ethylene groups in their framework has been synthesized by Xia et al., in the presence of bis(triethoxysilyl)ethylene (BTEE) precursor and CTAB template[34].
This entry is adapted from the peer-reviewed paper 10.3390/nano10112122
References
- Xin Du; Junhui He; Spherical silica micro/nanomaterials with hierarchical structures: Synthesis and applications. Nanoscale 2011, 3, 3984-4002, 10.1039/c1nr10660k.
- Kolbe, G. Das komplexehemische Verhalten der Kieselsure. Ph.D. Thesis, Friedrich-Schiller Universität, Jena, Germany, 1956.
- Werner Stöber; Arthur Fink; Ernst Bohn; Controlled growth of monodisperse silica spheres in the micron size range. Journal of Colloid and Interface Science 1968, 26, 62-69, 10.1016/0021-9797(68)90272-5.
- Zhe Chen; Bo Peng; Jia-Qiong Xu; Xue-Chen Xiang; Dong-Fang Ren; Taiqun Yang; Shi-Yu Ma; Kun Zhang; Qiming Chen; A non-surfactant self-templating strategy for mesoporous silica nanospheres: beyond the Stöber method. Nanoscale 2020, 12, 3657-3662, 10.1039/c9nr10939k.
- Sajo P. Naik; S. P. Elangovan; Tatsuya Okubo; Igor Sokolov; Morphology Control of Mesoporous Silica Particles. The Journal of Physical Chemistry C 2007, 111, 11168-11173, 10.1021/jp072184a.
- T. Martin; A. Galarneau; Francesco Di Renzo; F. Fajula; D. Plee; Morphological Control of MCM-41 by Pseudomorphic Synthesis. Angewandte Chemie International Edition 2002, 41, 2590-2592, 10.1002/1521-3773(20020715)41:14<2590::aid-anie2590>3.0.co;2-3.
- Sangyun Lim; Alpana Ranade; Guoan Du; Lisa D. Pfefferle; Gary L. Haller; Pseudomorphic Synthesis of Large-Particle Co−MCM-41. Chemistry of Materials 2006, 18, 5584-5590, 10.1021/cm061342s.
- Bruno Alonso; Christian Clinard; Minique Durand; Emmanuel Veron; Dominique Massiot; New routes to mesoporous silica-based spheres with functionalised surfaces. Chem. Commun. 2005, 13, 1746-1748, 10.1039/b418191c.
- Toshiyuki Yokoi; Takumi Karouji; Seigo Ohta; Junko N. Kondo; Takashi Tatsumi; Synthesis of Mesoporous Silica Nanospheres Promoted by Basic Amino Acids and their Catalytic Application. Chemistry of Materials 2010, 22, 3900-3908, 10.1021/cm9037846.
- Zhen-An Qiao; Ling Zhang; Mingyi Guo; Yunling Liu; Qisheng Huo; Synthesis of Mesoporous Silica Nanoparticles via Controlled Hydrolysis and Condensation of Silicon Alkoxide. Chemistry of Materials 2009, 21, 3823-3829, 10.1021/cm901335k.
- Meihua Yu; Liang Zhou; Jun Zhang; Pei Yuan; Peter Thorn; Wenyi Gu; Chengzhong Yu; A simple approach to prepare monodisperse mesoporous silica nanospheres with adjustable sizes. Journal of Colloid and Interface Science 2012, 376, 67-75, 10.1016/j.jcis.2012.03.014.
- Asep Bayu Dani Nandiyanto; Soon-Gil Kim; Ferry Iskandar; Kikuo Okuyama; Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters. Microporous and Mesoporous Materials 2009, 120, 447-453, 10.1016/j.micromeso.2008.12.019.
- Vivek Polshettiwar; Dong Kyu Cha; Xixiang Zhang; Jean-Marie Basset; High-Surface-Area Silica Nanospheres (KCC-1) with a Fibrous Morphology. Angewandte Chemie International Edition 2010, 49, 9652-9656, 10.1002/anie.201003451.
- Katsunori Kosuge; Tatsuro Murakami; Nobuyuki Kikukawa; Makoto Takemori; Direct Synthesis of Porous Pure and Thiol-Functional Silica Spheres through the S+X-I+Assembly Pathway. Chemistry of Materials 2003, 15, 3184-3189, 10.1021/cm030225j.
- Katsunori Kosuge; Puyam S. Singh; Rapid Synthesis of Al-Containing Mesoporous Silica Hard Spheres of 30−50 μm Diameter. Chemistry of Materials 2001, 13, 2476-2482, 10.1021/cm000623b.
- Jinliang Liu; Chunyan Li; Fuyou Li; Fluorescence turn-on chemodosimeter-functionalized mesoporous silica nanoparticles and their application in cell imaging. Journal of Materials Chemistry 2011, 21, 7175-7181, 10.1039/c1jm10803d.
- † Robert I. Nooney; ‡ Dhanasekaran Thirunavukkarasu; § Yimei Chen; § And Robert Josephs; † Agnes E. Ostafin; Synthesis of Nanoscale Mesoporous Silica Spheres with Controlled Particle Size. Chemistry of Materials 2002, 14, 4721-4728, 10.1021/cm0204371.
- Katsunori Kosuge; Nobuyuki Kikukawa; Makoto Takemori; One-Step Preparation of Porous Silica Spheres from Sodium Silicate Using Triblock Copolymer Templating. Chemistry of Materials 2004, 16, 4181-4186, 10.1021/cm0400177.
- Tae‐Wan Kim; Po-Wen Chung; Victor S.-Y. Lin; Facile Synthesis of Monodisperse Spherical MCM-48 Mesoporous Silica Nanoparticles with Controlled Particle Size. Chemistry of Materials 2010, 22, 5093-5104, 10.1021/cm1017344.
- Qianjun He; Xiangzhi Cui; Fangming Cui; Limin Guo; Jianlin Shi; Size-controlled synthesis of monodispersed mesoporous silica nano-spheres under a neutral condition. Microporous and Mesoporous Materials 2009, 117, 609-616, 10.1016/j.micromeso.2008.08.004.
- G.V. Rama Rao; G.P. López; J. Bravo; H. Pham; A.K. Datye; H.F. Xu; T.L. Ward; Monodisperse Mesoporous Silica Microspheres Formed by Evaporation-Induced Self Assembly of Surfactant Templates in Aerosols. Advanced Materials 2002, 14, 1301-1304, 10.1002/1521-4095(20020916)14:18<1301::aid-adma1301>3.0.co;2-t.
- Wen Hua Fu; Yejun Guan; Yi Meng Wang; Ming-Yuan He; A facile synthesis of monodispersed mesoporous silica nanospheres with Pm3n structure. Microporous and Mesoporous Materials 2016, 220, 168-174, 10.1016/j.micromeso.2015.09.004.
- † Qisheng Huo; † Jianglin Feng; § And Ferdi Schüth; † Galen D. Stucky; Preparation of Hard Mesoporous Silica Spheres. Chemistry of Materials 1997, 9, 14-17, 10.1021/cm960464p.
- Yu Han; Jackie Y. Ying; Generalized Fluorocarbon-Surfactant-Mediated Synthesis of Nanoparticles with Various Mesoporous Structures. Angewandte Chemie International Edition 2005, 44, 288-292, 10.1002/anie.200460892.
- Johannes Kobler; Karin Möller; Thomas Bein; Colloidal Suspensions of Functionalized Mesoporous Silica Nanoparticles. ACS Nano 2008, 2, 791-799, 10.1021/nn700008s.
- Hironori Yamada; Chihiro Urata; Yuko Aoyama; Shimon Osada; Yusuke Yamauchi; Kazuyuki Kuroda; Preparation of Colloidal Mesoporous Silica Nanoparticles with Different Diameters and Their Unique Degradation Behavior in Static Aqueous Systems. Chemistry of Materials 2012, 24, 1462-1471, 10.1021/cm3001688.
- Johannes Köbler; Thomas Bein; Porous Thin Films of Functionalized Mesoporous Silica Nanoparticles. ACS Nano 2008, 2, 2324-2330, 10.1021/nn800505g.
- Stefan Datz; Hanna Engelke; Constantin V. Schirnding; Linh Nguyen; Thomas Bein; Lipid bilayer-coated curcumin-based mesoporous organosilica nanoparticles for cellular delivery. Microporous and Mesoporous Materials 2016, 225, 371-377, 10.1016/j.micromeso.2015.12.006.
- Kun Zhang; Lang-Lang Xu; Jin-Gang Jiang; Nathalie Calin; Koon-Fung Lam; San-Jun Zhang; Hai-Hong Wu; Guang-Dong Wu; Belén Albela; Laurent Bonneviot; et al. Facile Large-Scale Synthesis of Monodisperse Mesoporous Silica Nanospheres with Tunable Pore Structure. Journal of the American Chemical Society 2013, 135, 2427-2430, 10.1021/ja3116873.
- Ye-Jun Yu; Jun-Ling Xing; Jun-Ling Pang; Shu-Hua Jiang; Koon-Fung Lam; Tai-Qun Yang; Qing-Song Xue; Kun Zhang; Peng Wu; Facile Synthesis of Size Controllable Dendritic Mesoporous Silica Nanoparticles. ACS Applied Materials & Interfaces 2014, 6, 22655-22665, 10.1021/am506653n.
- Rahul P. Bagwe; Lisa R. Hilliard; Weihong Tan; Surface Modification of Silica Nanoparticles to Reduce Aggregation and Nonspecific Binding. Langmuir 2006, 22, 4357-4362, 10.1021/la052797j.
- Kai Cheng; Christopher C. Landry; Diffusion-Based Deprotection in Mesoporous Materials: A Strategy for Differential Functionalization of Porous Silica Particles. Journal of the American Chemical Society 2007, 129, 9674-9685, 10.1021/ja070598b.
- Vivian Rebbin; Michaela Jakubowski; Steffen Pötz; Michael Fröba; Synthesis and characterisation of spherical periodic mesoporous organosilicas (sph-PMOs) with variable pore diameters. Microporous and Mesoporous Materials 2004, 72, 99-104, 10.1016/j.micromeso.2004.04.018.
- Yongde Xia; Robert Mokaya; High surface area ethylene-bridged mesoporous and supermicroporous organosilica spheres. Microporous and Mesoporous Materials 2005, 86, 231-242, 10.1016/j.micromeso.2005.06.040.
