Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Many intrinsic and extrinsic factors influences the female reproductive tract microbiota (FRTM) that directly affects the reproductive health. It is believed that FRTM dominated by Lactobacilli may play an essential role in obstetric health beyond the woman’s intimate comfort and well-being. Women with altered microbiota may face numerous health-related issues. Altered microbiota can be manipulated and restored to their original shape to re-establish normal reproductive health.
- Lactobacillus
- lactic acid
- microbiota
- reproductive health
- vagina
1. Introduction
The findings of Human Microbiome Project (HMP) proved the existence of a diverse microbial population and their eight million distinctive genetic elements throughout the human body, having elementary roles in human health and diseases [1]. It has been reported that about 30 trillion human cells/body, along with an estimated 39 trillion microbial cells, which includes bacteria, archaea, fungi, algae, and viruses, live on and inside the body [2][3]. Our “microbiota” comprises an assorted population of bacteria, viruses, fungi, and other unicellular organisms living in or on humans. The collection of all the genes within these microscopic organisms is known as the human “microbiome” [4]. The microbiome is not only the collection of genes, but also includes the structural elements, metabolites/signal molecules, and the surrounding environmental conditions [5]. Microbiomes have been studied intensively since the nineteenth century and are traditionally characterized using cultivation methods [1]. Recent findings have suggested the direct link of body microbiota in the regulation of various female reproductive complications such as endometriosis, PCOS (polycystic ovary syndrome), RPL (recurrent pregnancy loss), pregnancy complications, gynecologic cancer, and infertility [6][7][8]. Recent studies have also suggested that “vaginal seeding” (Wiping of infant’s body including mouth and face with its mother vaginal fluid) is helpful to restore the microbiome and the development of immunity, especially in the C-section delivery, where the newborn is devoid of direct exposure to the vaginal secretion of the mother [9]. Few studies consider the vaginal microbiome as a tool to predict the success of IVF/assisted reproductive technology [10]. An in-depth profile of the microbiome has recently been generated with the appearance of advanced molecular technology that demonstrated greater microbial diversity than previously recognized [11]. Interestingly, among the body’s microbiome, the specific female reproductive tract (FRT) houses nine percent of the total microbial population of the entire body [12]. Most investigations have been focused to study the microbiota of the lower reproductive tract (LRT) [13]. However, recent investigations proved the presence of a diverse microbial ecology in the endometrium and other locations of the upper reproductive tract (URT) [14][15]. The microbial burden is progressively reduced from reproductive tract’s lower to upper portion [16][17]. The composition of LRT microbiota changes during the entire female’s lifecycle from childhood to reproductive age and up to menopause [18]. Hormonal changes in a woman are one of the critical factors that regulates the microbiota configuration at different stages of a woman’s life [19]. The cervicovaginal microbiota is extensively screened and categorized into at least six types, named community state types (CSTs) [13][20]. Human females have Lactobacillus spp. as the predominant group in the pool of FRTM, while in the other mammals, the Lactobacillus population is merely more than 1% [21][22]. Lactic acid, the predominant metabolic byproduct of Lactobacillus when glycogen serves as the primary substrate, resulted in an exceptionally low pH (≤4.5) in the lower reproductive tract [23]. Certain Community State Types (CSTs) dominated by Lactobacillus spp., principally L. crispatus, are more correlated to reproductive eubiosis than CSTs having less abundant Lactobacilli [13]. The optimum composition of the FRTM, dominated by Lactobacillus spp. and acidic pH, diversely benefits the host. Several external and host-associated factors may disturb the optimum composition of normal microbiota, which leads to compromised reproductive health and severe gynecological conditions, including BV, sterility, and preterm delivery, and are a more significant threat of sexually transmitted infections (STIs) [24]. Many strategies have been projected to effectively restore optimum balance in the FRTM, including antibiotics, probiotics, hormone replacement therapy (HRT), vaginal fluid transplant, and a combination of any two or more approaches [25][26].
2. Microbiota of Female Reproductive Tract
Distinct microbial communities exist throughout the female reproductive tract (FRT), starting from the vaginal opening to the placenta [27][28]. The lower reproductive tract (LRT) comprises the vagina and cervix together, known as the cervicovagina. In most recent studies, cervicovaginal microbiota are generally studied together [29]. The cervicovaginal microbiota resides in and on the epithelium’s outermost layer. In the LRT, a healthy cervicovagina demonstrates the dominancy of Lactobacillus spp. (107–109 Lactobacilli/gram of vaginal fluid) that accounts for up to 95% load of the total bacterial population residing in the entire RT [30][31]. The cervicovaginal microbiota of reproductive-aged females has been categorized into five major clusters, termed community state types (CSTs). Out of five, four CSTs exhibited dominancy of Lactobacillus spp. CST-I is dominated by L. crispatus, whereas CST-II, CST-III, and CST-V show dominancy of L. gasseri, L. iners, and L. jensenii, respectively. The fifth one, CST-IV, has a lower density of Lactobacillus spp. [13]. CTS-IV is categorized into two subgroups, A and B. Subgroup IV-A comprises a modest population of Lactobacillus spp. and other species, i.e., A. vaginae, G. vaginalis, and Prevotella spp. Subgroup IV-B comprises microbial species including A. vaginae, Leptotrichia spp., and Mobiluncus spp. [20][27]. Interestingly, it has been observed a shifting of different CST populations in different parts of the reproductive tracts of women [32] (Table 1).
The upper reproductive tract (URT) comprises the endocervix, endometrium, uterine cavity, fallopian tubes, ovary, peritoneal fluid, and placenta. The existence of bacteria in the URT remains controversial and for a long time has been considered a germ-free region. Recent studies have challenged this “sterile womb” dogma by proving the colonization of bacteria in the URT even in the absence of any infection [11][16]. The origination of microbiota identified in the URT is still unclear. It is hypothesized that they ascend from the vagina probably due to spontaneous uterine contractions, which are most intense during ovulation and orgasms [33]. Bacterial load gradually decreases from the LRT to the URT. Uterine bacteria were estimated to be about 10,000 times lesser than that of the cervicovagina, and the most dominant ones were Prevotella spp., L. iners, and L. crispatus [16].
Table 1. Comparative description of different Community State Types (CSTs) on the basis of prominent organism, pH, Nugent score, pregnancy status, major cell type, and reproductive health.
| Community State Type | Prominent Organism (% Dominancy) [13] | Median pH (All Ethnic Groups) [13] | Nugent Score [13][27] | %Nonpregnant Women [34] | %Normal Pregnancy [34] | Epithelial Cells [35] | Reproductive Health |
|---|---|---|---|---|---|---|---|
| CST-I | L. crispatus (26.2%) | 4.0 ± 0.3 (Lowest pH) | Lowest Nugent score (0–3) | 17 | 38.1 | Mature squamous cells (MSCs) | Healthy condition |
| CST-II | L. gasseri (6.3%) | 5.0 ± 0.7 | Nugent score (4–6) | 8.9 | 4.3 | MSCs | Healthy condition |
| CST-III | L. iners (34.1%) | 4.4 ± 0.6 | Low Nugent score (0–3) | 35.2 | 51.8 | MSCs/# Immature parabasal cells | Healthy condition (less stable or more in transition) [36] |
| CST-IVA | No particular prevailing species Different levels of L. inners or other Lactobacillus spp., with low proportions of Anaerococcus, Corynebacterium Finegoldia, Streptococcus [27] | 5.3 ± 0.6 (highest pH) CST-IVB has higher pH than CST-IVA | Relatively lower Nugent scores than IV-B (7–10) | 10.4 | 3.6 | MSCs/# Immature parabasal cells | Risk associated with PTB and obstetrical complications [34][37] Associated with HPV infection, CIN, and HIV acquisition [38] Dominant in postpartum stage [39] CST-IV are risk factors for BV [32] |
| CST-IVB | No particular prevailing species Comparatively high levels of Atopobium, Gardnerella, Mobiluncus, Peptoniphilus, Sneathia, Prevotella, and several other taxa of BVAB [20][27] | Contains some of the BV-associated bacteria (BVAB) and is often associated with highest Nugent scores (7–10) | 28.5 | 2.2 | |||
| CST-V | L. jensenii (5.3%) | 4.7 ± 0.4 | Nugent score (4–6) | MSCs | Healthy condition |
# Desquamative inflammatory vaginosis.
Additional groups steadily recognized were Bifidobacterium, Corynebacterium, Staphylococcus, and Streptococcus [40]. Lactobacillus is the most dominant group that constantly exists in the URT. Endometrial fluid may be broadly categorized into two clusters: (i) the Lactobacillus-dominated (LD) cluster and (ii) non-Lactobacillus-dominated (NLD) clusters. Aagaard et al. proposed that the placenta is a house of metabolically active and less-abundant microbiota that are composed mainly of nonpathogens of the Bacteroidetes, Proteobacteria, Firmicutes, Fusobacteria, and Tenericutes phyla [41]. The microbiota of healthy female fallopian tubes has yet to be well characterized. Pelzer et al. identified Enterococcus sp. and Staphylococcus sp., However, Lactobacillus sp. is the most abundant microflora present in a fallopian tube, along with other sp., including Pseudomonads, Propionibacterium, and Prevotella [28]. Recently Chen et al. identified a variety of microbiomes as a signature, primarily of Facklamia, Erysipelothrix, and Pseudomonas in the fallopian tube and Morganella, Pseudomonas, Sphingobium, and Vagococcus in peritoneal fluid [16].
3. Factors That Influence the Composition of FRTM
Several endogenous and environmental factors directly influence and alter the FRTM composition and cervicovaginal milieu (Figure 1). A starch-rich diet increases glycogen levels in the vagina, thus creating a favorable environment to proliferate lactobacilli [21]. The prepubic cervicovaginal microbiota are rated as relatively stable build-ups of aerobes, anaerobes, and intestinal microbial communities, which primarily shows the dominancy of anaerobes, i.e., the Enterobacteriaceae and/or Staphylococcacee family [42]. In the active reproductive age, due to the elevated level of estrogen, lactic acid bacteria colonize the vagina, which contributes to the acidification of the cervicovaginal region by discharging principally lactic acid and some other organic acids [43]. The dominancy of Lactobacillus is maintained throughout the reproductive phase. During the menopausal stage, the estrogen level drops, a thinner vaginal epithelium containing low glycogen and reduced mucin secretion results in a less dominant Lactobacillus population, and hence an elevated vaginal pH (>5), rendering the female genitourinary tract more susceptible to infections [44]. In pregnant women, the absence of menses, an increased level of sex hormones (placental estrogen), and a thicker vaginal mucosa stuffed with glycogen leads to increased glycogen metabolism and reduced pH (<4.5) [45]. The low vaginal pH, due to lactic acid production, may contribute to the lower bacterial diversity and greater dominancy of Lactobacillus sp., hence reducing the risk of BV and other infections during pregnancy [34].
It has been reported that different races or ethnic groups have different microbial compositions due to the diversity in their genetic constitution [46]. Sexual behavior and the lifestyle of the host are the leading factors that influence the FRTM. Homosexual relationships, unprotected sex, and having multiple, new, or numerous male partners negatively affect vaginal homeostasis [47][48]. Additionally, reproductive hygiene, the type of contraception, and antibiotic treatments also have directly influenced the FRTM. It has been also reported that detergent-based nonspecific vaginal contraceptives can also adversely affect normal microbiota of reproductive tract [49]. Hormonal contraceptives can stimulate the colonization of beneficial lactobacilli and are supposed to have a role in the stabilization of balanced vaginal microbiota and reduced risk of BV [50]. It is observed that broad-spectrum antimicrobials can adversely affect the harmful bacteria as well as reduce the number of beneficial bacteria in the RT [51].
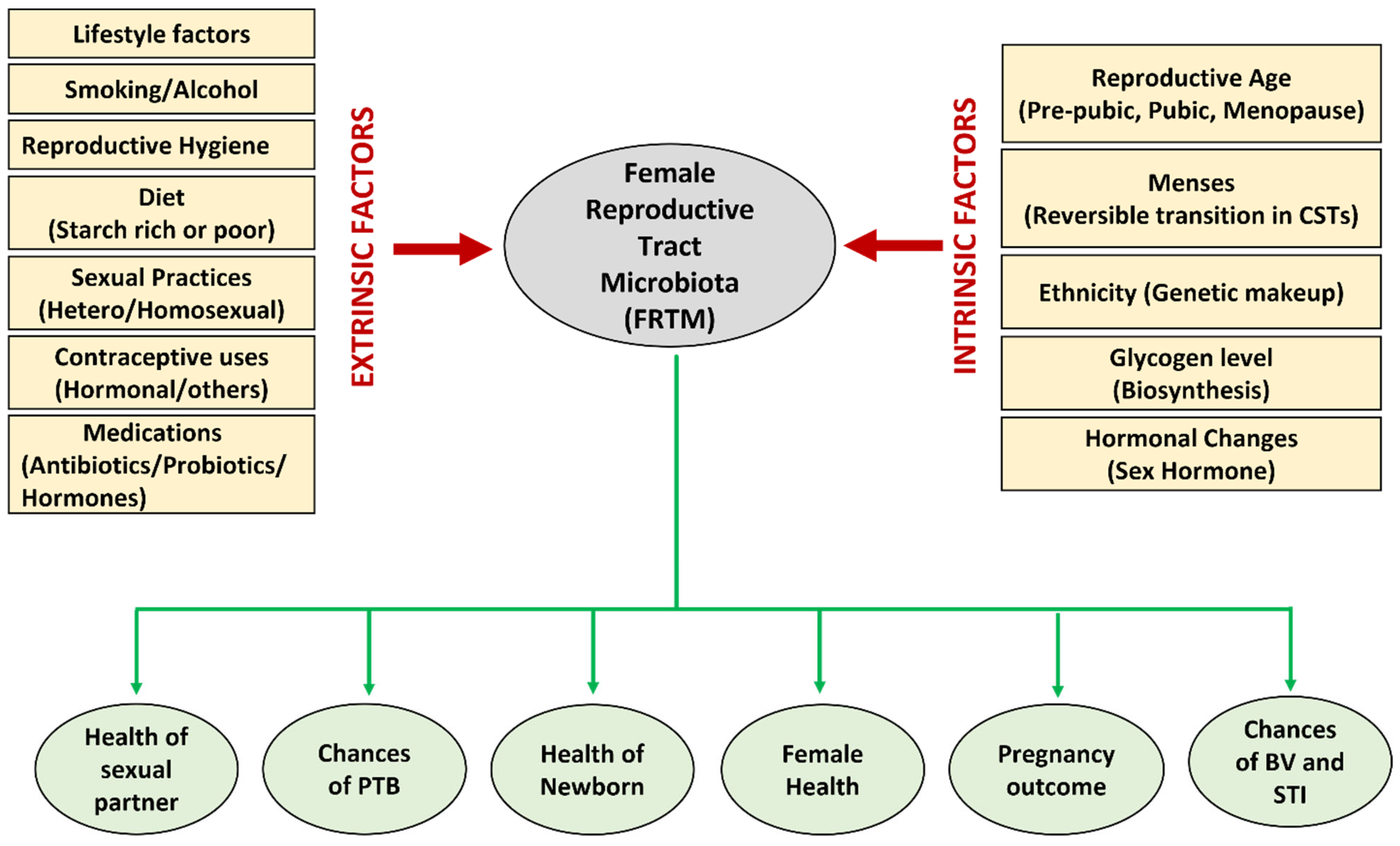
Figure 1. Various extrinsic and intrinsic factors that influence the composition of the FRTM, and various aspects of reproductive health directly or indirectly affected by the microbiota.
This entry is adapted from the peer-reviewed paper 10.3390/life13061313
References
- Methé, B.A.; Nelson, K.E.; Pop, M.; Creasy, H.H.; Giglio, M.G.; Huttenhower, C.; Gevers, D.; Petrosino, J.F.; Abubucker, S.; Mannon, P.J.; et al. A framework for human microbiome research. Nature 2012, 486, 215–221.
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLOS Biol. 2016, 14, e1002533.
- Franasiak, J.M.; Scott, R.T. Introduction: Microbiome in human reproduction. Fertil. Steril. 2015, 104, 1341–1343.
- Yang, J. The Human Microbiome Project: Extending the Definition of What Constitutes a Human. National Human Genome Research Institute; 2012. Available online: https://www.genome.gov/27549400/the-human-microbiome-project-extending-the-definition-of-what-constitutes-a-human (accessed on 10 March 2023).
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103.
- Peuranpää, P.; Holster, T.; Saqib, S.; Kalliala, I.; Tiitinen, A.; Salonen, A.; Hautamäki, H. Female reproductive tract microbiota and recurrent pregnancy loss: A nested case-control study. Reprod. Biomed. Online 2022, 45, 1021–1031.
- Chambers, L.M.; Bussies, P.; Vargas, R.; Esakov, E.; Tewari, S.; Reizes, O.; Michener, C. The Microbiome and Gynecologic Cancer: Current Evidence and Future Opportunities. Curr. Oncol. Rep. 2021, 23, 92.
- Ser, H.L.; Au Yong, S.J.; Shafiee, M.N.; Mokhtar, N.M. Current Updates on the Role of Microbiome in Endometriosis: A Narrative Review. Microorganisms 2023, 11, 360.
- Hourigan, S.K.; Dominguez-Bello, M.G.; Mueller, N.T. Can maternal-child microbial seeding interventions improve the health of infants delivered by Cesarean section? Cell Host Microbe 2022, 30, 607–611.
- Schoenmakers, S.; Laven, J. The vaginal microbiome as a tool to predict IVF success. Curr. Opin. Obstet. Gynecol. 2020, 32, 169–178.
- Moreno, I.; Simon, C. Deciphering the effect of reproductive tract microbiota on human reproduction. Reprod. Med. Biol. 2018, 18, 40–50.
- Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; Deal, C.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323.
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687.
- Walther-António, M.R.S.; Chen, J.; Multinu, F.; Hokenstad, A.; Distad, T.J.; Cheek, E.H.; Keeney, G.L.; Creedon, D.J.; Nelson, H.; Mariani, A.; et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016, 8, 122.
- Miles, S.M.; Hardy, B.L.; Merrell, D. Investigation of the microbiota of the reproductive tract in women undergoing a total hysterectomy and bilateral salpingo-oopherectomy. Fertil. Steril. 2017, 107, 813–820.e1.
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875.
- Younes, J.A.; Lievens, E.; Hummelen, R.; van der Westen, R.; Reid, G.; Petrova, M.I. Women and Their Microbes: The Unexpected Friendship. Trends Microbiol. 2017, 26, 16–32.
- Hillier, S.L.; Lau, R.J. Vaginal Microflora in Postmenopausal Women Who Have Not Received Estrogen Replacement Therapy. Clin. Infect. Dis. 1997, 25, S123–S126.
- Kaur, H.; Merchant, M.; Haque, M.M.; Mande, S.S. Crosstalk Between Female Gonadal Hormones and Vaginal Microbiota Across Various Phases of Women’s Gynecological Lifecycle. Front. Microbiol. 2020, 11, 551.
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.E.; Zhong, X.; Koenig, S.S.K.; Fu, L.; Ma, Z.; Zhou, X.; et al. Temporal Dynamics of the Human Vaginal Microbiota. Sci. Transl. Med. 2012, 4, 132ra52.
- Miller, E.A.; Beasley, D.E.; Dunn, R.R.; Archie, E.A. Lactobacilli Dominance and Vaginal pH: Why Is the Human Vaginal Microbiome Unique? Front. Microbiol. 2016, 7, 1936.
- Spear, G.T.; French, A.L.; Gilbert, D.; Zariffard, M.R.; Mirmonsef, P.; Sullivan, T.H.; Spear, W.W.; Landay, A.; Micci, S.; Lee, B.-H.; et al. Human α-amylase Present in Lower-Genital-Tract Mucosal Fluid Processes Glycogen to Support Vaginal Colonization by Lactobacillus. J. Infect. Dis. 2014, 210, 1019–1028.
- Mirmonsef, P.; Hotton, A.L.; Gilbert, D.; Burgad, D.; Landay, A.; Weber, K.M.; Cohen, M.; Ravel, J.; Spear, G.T. Free Glycogen in Vaginal Fluids Is Associated with Lactobacillus Colonization and Low Vaginal pH. PLoS ONE 2014, 9, e102467.
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792.
- Gliniewicz, K.; Schneider, G.M.; Ridenhour, B.J.; Williams, C.J.; Song, Y.; Farage, M.A.; Miller, K.; Forney, L.J. Comparison of the Vaginal Microbiomes of Premenopausal and Postmenopausal Women. Front. Microbiol. 2019, 10, 193.
- Deng, Z.-L.; Gottschick, C.; Bhuju, S.; Masur, C.; Abels, C.; Wagner-Döbler, I. Metatranscriptome Analysis of the Vaginal Microbiota Reveals Potential Mechanisms for Protection against Metronidazole in Bacterial Vaginosis. mSphere 2018, 3, e00262-18.
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal Microbiome: Rethinking Health and Disease. Annu. Rev. Microbiol. 2012, 66, 371–389.
- Pelzer, E.S.; Willner, D.; Buttini, M.; Hafner, L.M.; Theodoropoulos, C.; Huygens, F. The fallopian tube microbiome: Implications for reproductive health. Oncotarget 2018, 9, 21541–21551.
- Elovitz, M.A.; Gajer, P.; Riis, V.; Brown, A.G.; Humphrys, M.S.; Holm, J.B.; Ravel, J. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat. Commun. 2019, 10, 1305.
- Delaney, M.L.; Onderdonk, A.B. Nugent score related to vaginal culture in pregnant women. Obstet. Gynecol. 2001, 98, 79–84.
- Srinivasan, S.; Liu, C.; Mitchell, C.M.; Fiedler, T.L.; Thomas, K.K.; Agnew, K.J.; Marrazzo, J.M.; Fredricks, D.N. Temporal Variability of Human Vaginal Bacteria and Relationship with Bacterial Vaginosis. PLoS ONE 2010, 5, e10197.
- Bradshaw, C.S.; Walker, J.; Fairley, C.K.; Chen, M.Y.; Tabrizi, S.N.; Donovan, B.; Kaldor, J.M.; McNamee, K.; Urban, E.; Walker, S.; et al. Prevalent and incident bacterial vaginosis are associated with sexual and contraceptive behaviours in young Australian women. PLoS ONE 2013, 8, e57688.
- Bulletti, C.; de Ziegler, D.; Polli, V.; Diotallevi, L.; Del Ferro, E.; Flamigni, C. Uterine contractility during the menstrual cycle. Hum. Reprod. 2000, 15 (Suppl. S1), 81–89.
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765.
- Paavonen, J.; Brunham, R.C. Bacterial Vaginosis and Desquamative Inflammatory Vaginitis. N. Engl. J. Med. 2018, 379, 2246–2254.
- Petrova, M.I.; Reid, G.; Vaneechoutte, M.; Lebeer, S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2016, 25, 182–191.
- Anahtar, M.N.; Gootenberg, D.B.; Mitchell, C.M.; Kwon, D.S. Cervicovaginal Microbiota and Reproductive Health: The Virtue of Simplicity. Cell Host Microbe 2018, 23, 159–168.
- Curty, G.; Costa, R.L.; Siqueira, J.D.; Meyrelles, A.I.; Machado, E.S.; Soares, E.A.; Soares, M.A. Analysis of the cervical microbiome and potential biomarkers from postpartum HIV-positive women displaying cervical intraepithelial lesions. Sci. Rep. 2017, 7, 17364.
- DiGiulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyell, D.J.; Robaczewska, A.; Sun, C.L.; Goltsman, D.S.A.; Wong, R.J.; Shaw, G.; et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065.
- Tao, X.; Franasiak, J.M.; Zhan, Y.; Scott, R.T.; Rajchel, J.; Bedard, J.; Newby, R.; Treff, N.R.; Chu, T. Characterizing the endometrial microbiome by analyzing the ultra-low bacteria from embryo transfer catheter tips in IVF cycles: Next generation sequencing (NGS) analysis of the 16S ribosomal gene. Hum. Microbiome J. 2017, 3, 15–21.
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014, 6, 237ra65.
- Hill, G.B.; Claire, K.K.S.; Gutman, L.T. Anaerobes Predominate Among the Vaginal Microflora of Prepubertal Girls. Clin. Infect. Dis. 1995, 20 (Suppl. S2), S269–S270.
- Linhares, I.M.; Summers, P.R.; Larsen, B.; Giraldo, P.C.; Witkin, S.S. Contemporary perspectives on vaginal pH and lactobacilli. Am. J. Obstet. Gynecol. 2011, 204, 120.e1–120.e5.
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Fadrosh, D.; Chang, K.R.; Silver, M.I.S.; Viscidi, R.P.; Burke, A.E.; Ravel, J.; Gravitt, P.E. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 2018, 25, 1321–1330.
- Cruickshank, R.; Sharman, A. The biology of the vagina in the human subject. II The bacterial flora and secretion of the vagina at various age-periods and their relations to glycogen in the vaginal epitehlial. BJOG Int. J. Obstet. Gynaecol. 1934, 41, 190–207.
- Schreiber, C.A.; Meyn, L.A.; Creinin, M.D.; Barnhart, K.T.; Hillier, S.L. Effects of Long-Term Use of Nonoxynol-9 on VaginalFlora. Obstet. Gynecol. 2006, 107, 136–143.
- Fethers, K.A.; Fairley, C.K.; Hocking, J.; Gurrin, L.; Bradshaw, C.S. Sexual Risk Factors and Bacterial Vaginosis: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2008, 47, 1426–1435.
- Forcey, D.S.; Vodstrcil, L.A.; Hocking, J.S.; Fairley, C.K.; Law, M.; McNair, R.P.; Bradshaw, C.S. Factors Associated with Bacterial Vaginosis among Women Who Have Sex with Women: A Systematic Review. PLoS ONE 2015, 10, e0141905.
- Jain, A.; Lal, N.; Kumar, L.; Verma, V.; Kumar, R.; Kumar, L.; Singh, V.; Mishra, R.K.; Sarswat, A.; Jain, S.K.; et al. Novel Trichomonacidal Spermicides. Antimicrob. Agents Chemother. 2011, 55, 4343–4351.
- Vodstrcil, L.A.; Hocking, J.S.; Law, M.; Walker, S.; Tabrizi, S.N.; Fairley, C.K.; Bradshaw, C.S. Hormonal Contraception Is Associated with a Reduced Risk of Bacterial Vaginosis: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e73055.
- Sobel, J.D.; Wiesenfeld, H.C.; Martens, M.; Danna, P.; Hooton, T.M.; Rompalo, A.; Sperling, M.; Livengood, C.; Horowitz, B.; Von Thron, J.; et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N. Engl. J. Med. 2004, 351, 876–883.
This entry is offline, you can click here to edit this entry!
