Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Coumarins are secondary plant metabolites widely distributed in higher plants, bacteria, fungi, and sponges. This great structural diversity of these natural compounds and their synthesized derivatives enables their wide range of pharmacological activities, such as antioxidant; antibacterial; antifungal; anti-human immunodeficiency infection; anti-tubercular; and anti-cancer activities. There are also many reports about their effectiveness against plant pathogenic pests (phytopathogenic fungi, bacteria, nematodes, and insects).
- coumarins
- pesticides
1. Introduction
The control of fungal pathogens and pests has vital importance for the protection of crops and food provision worldwide. Organic compounds are still the major active components of plant protection products. Although pesticides in agronomy exert powerful effects on disease control management, the long-term their unreasonable use led to serious environmental problems inducing successive pesticide-resistant pathogen development, disrupting soil ecological balance, and causing environmental and human health. There are among the most common pollutants in one-fifth of the Earth’s land. Plant protection products pollute terrestrial and aquatic ecosystems, and every year millions of people are exposed to pesticides [1]. Daily exposure to pesticides has numerous consequences for human health. Pesticide exposure has numerous health consequences. Pesticides could induce tumors in the liver, lungs, stomach, kidneys, skin, and stomach [2][3]. Also, exposure to pesticides has been associated with dermatological, gastrointestinal, neurological, respiratory, reproductive, and endocrine symptoms of diseases [4]. Drug resistance and environmental and health hazards indicate the urgent need for novel active compounds. These compounds must be highly specific with a broad-spectrum mode of action, as well as environmentally and toxicologically acceptable [5]. In order to limit pesticide harmful effects, the European Parliament and the Council issued Directive 2009/128/EC that promotes integrated pest management with priority for plant protection products. This Directive has the fewest side effects on human health, non-target organisms, and the environment [6]. Coumarins are secondary plant metabolites widely distributed in higher plants, bacteria, fungi, and sponges [7]. Depending on the substitution of the 1-benzopyran-2-one skeleton, coumarins can be divided into several types: simple coumarins, furanocoumarins, pyranocoumarins, and other coumarins (Figure 1). This great structural diversity of these natural compounds and their synthesized derivatives enables their wide range of pharmacological activities, such as antioxidant [8][9][10]; antibacterial [11][12][13][14][15][16][17]; antifungal [18][19][20]; anti-human immunodeficiency virus (HIV) infection [21][22]; anti-tubercular [23]; cytotoxicity [24]; and anti-cancer activities [25][26][27][28][29].
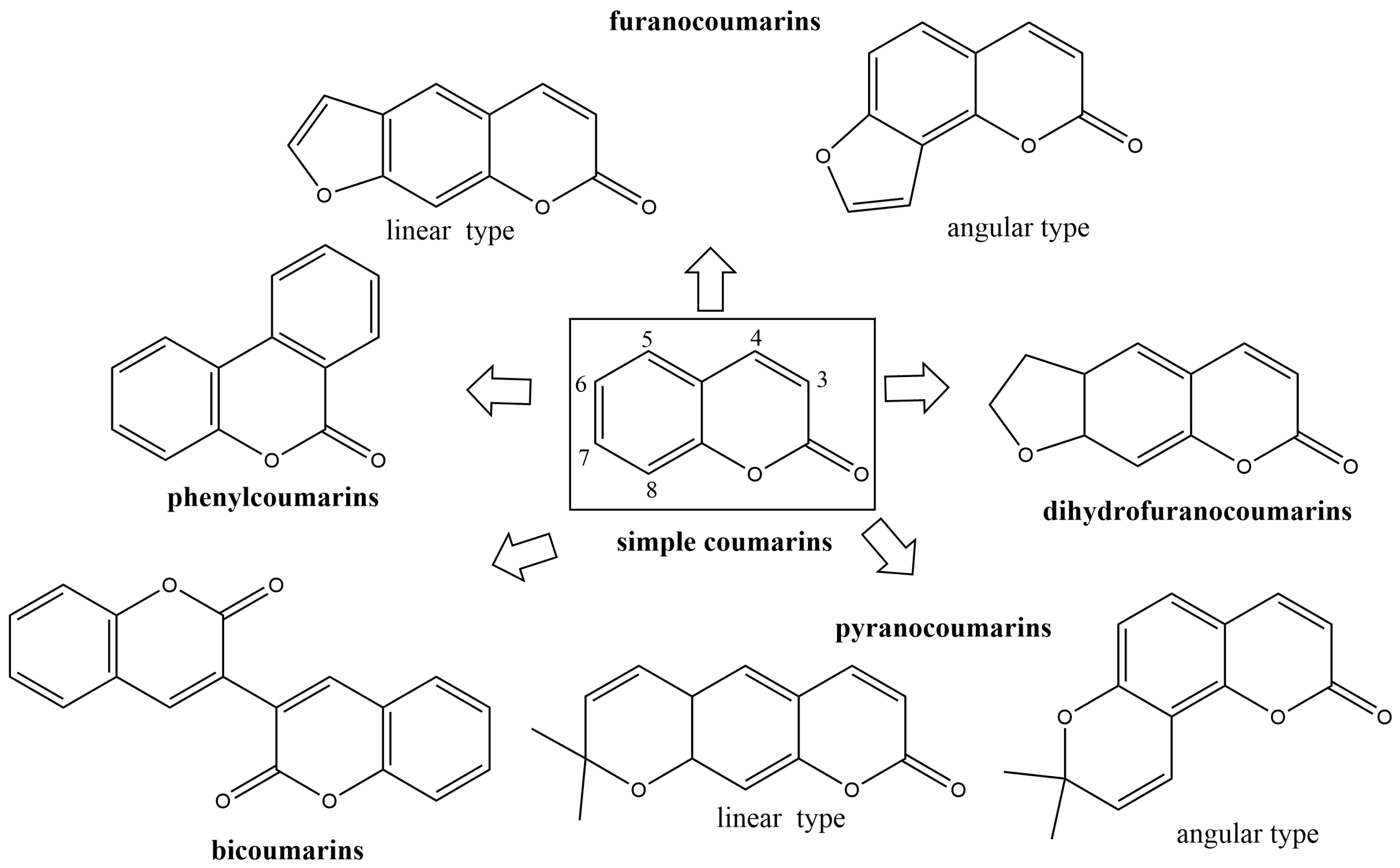
Figure 1. The main groups of natural coumarins.
There are also many reports about their effectiveness against plant pathogenic pests. These secondary metabolites are natural protection agents against environmental enemies and competing plants, therefore they are called allelochemicals. Allelochemicals are biocommunicators that act in a natural mixture of active components, while single compounds are not active [7][30][31]. Coumarin derivatives have been reported as strong agents against phytopathogen fungi, such as: Botrytis cinerea [32]; Moniliophthora perniciosa [33]; Colletotrichum gloeosporioides, Fusarium oxysporum, Valsa mali [34]; Macrophomina phaseolina and Sclerotinia sclerotiorum [35][36]. Coumarins have also antimicrobial potential against phytopathogens: Ralstonia solanacearum [37]; Agrobacterium tumefaciens [38]; Pseudomonas aeruginosa [39]. Nematicidal activity has been demonstrated for several simple coumarins, furanocoumarines, and dicoumarols, and their skeletons have been used for the development of new efficient nematicides against plant parasitic nematodes: Meloidogyne incognita, Ditylenchus destructor, Bursaphelenchus xylophilus, Bursaphelenchus mucronatus, and Aphelenchoides besseyi [40][41].
2. Naturally Occurring Coumarins and Their Role in Plants
2.1. Biosynthesis and Distribution of Coumarins in Nature
As a family of benzopyrones (1,2-benzopyrones or 2H-1-benzopyran-2-ones), coumarins are widely distributed throughout nature. The benzopyrone framework is an electron-rich system with favorable charge-transport properties. Therefore, they are characterized by UV light absorption, resulting in a characteristic blue fluorescence. Besides their role in iron mobilization and uptake by plant roots, natural coumarins have a role in environmental stress responses. Also, they participate in the defense against plant pathogens, acting as phytoanticipins, or phytoalexins, which are produced upon infection and are typically not present in healthy tissues. Their increased accumulation on plant tissue is a response to the application of a molecule that triggers the hypersensitivity response in the plant (elicitor) or plant hormones [42].
Since coumarins act as signaling molecules that regulate the interaction between commensals, pathogens, and plants, they could be used as biopesticides. Endophytes such as bacteria or fungi have the ability to produce some of the secondary metabolites. Thus, coumarin isofraxidin was synthesized by the fungal endophyte Biscognia uxiacylindrospora, and identified in host plants Siberian ginseng (Acanthopanax senticosus) and Apium graveolens [43]. Infection by pathogenic bacteria can induce the synthesis of coumarin compounds around plant roots and stems to immunize the plant against pathogen invasion and propagation. Inoculation of Arabidopsis thaliana by the plant pathogen Dickeya spp. strains induced coumarin accumulation and plant resistance to pathogens [44].
Treatment of ryegrass (Lolium multiflorum Lam.) with coumarins stimulates the colonization of beneficial flora in the root rhizospheric microbial community. Rhizosphere microorganisms enhance plant nutrient absorption, coordinate growth, and improve environmental adaptability [45]. The secretion of coumarins from Arabidopsis thaliana roots under soil iron deprivation stimulates the bacterial root microbiota to improve plant adaptation to iron-limiting soils [46]. A strain of Aspergillus synthesizes 4-hydroxycoumarin and dicoumarol [47][48].
Naturally occurring coumarins are mostly distributed in plants seeds, flowers, leaves, roots, and stems in more than 40 different families including Apiaceae, Rutaceae, Asteraceae, Fabaceae, Oleaceae, Moraceae, and Thymelaeaceae [49]. The natural coumarins are derivatives of 2H-1-benzopyran-2-one, and they are classified into six groups: simple coumarins; furanocoumarins (linear and angular type); dihidrofuranocoumarins; pyranocoumarins (linear and angular type); phenylcoumarins; and bicoumarins (Figure 1) [50]. Angiospermaes are rich in simple coumarins, followed by furanocoumarins and pyranocoumarins. The most diverse sources of coumarins are plants families Apiaceae and Rutaceae containing five different types of coumarin derivatives (simple coumarins, lineal furocoumarins, angular furocoumarins, lineal pyranocoumarins, and angular pyranocoumarins) [30].
Simple coumarins are derived by biosynthesis from shikimic acid, via cinnamic acid. They are the most common in all angiosperms, especially in Oleaceae and Asteraceae. A key step in the biosynthesis of simple coumarins is ortho-hydroxylation of cinnamates that branch off from lignin biosynthesis. The gene required for the production of feruloyl coenzyme A (CoA) is CCoAOMT1. It also participates in the biosynthesis of lignin and simple coumarin scopoletin in Arabidopsis roots. A key enzyme involved in the biosynthesis of simple coumarins is 2-oxoglutarate-dependent dioxygenase (2OGD), which is encoded by 2OGD genes. Thus, the gene AtF6_H1 encodes otho-hydroxylase activity to feruloyl coenzyme A, and its deficient mutation causes a significant reduction in scopolin accumulation. 2OGD gene RgC2′H formation of furanocoumarins in Ruta graveolenes [51]. Beta-glucosidase (BGLU) genes are regulatory genes responsible for coumarin biosynthesis in Melilotus species and differences in their expression result in coumarin content diversity among Melilotus species [52]. Coumarin biosynthesis genes are also activated after foliar pathogen infection to create a microbial soil-borne legacy that primes plants for defenses. Coumarin biosynthesis genes, such as root-specific transcription factors myb72 and f6′h1 are also activated in the plant Arabidopsis thaliana after foliar pathogen infection with downy mildew pathogen Hyaloperonospora arabidopsis (Hpa), for the creation of a microbial soil-borne legacy (SBL) that primes plants for defenses [53]. Since scopoletin selectively inhibits the soil-borne fungal pathogens Fusarium oxysporum and Verticillium dahliae, the study of Stringlis et al. [54] has shown that during infection, probiotic root-associated microbes stimulate MYB72-dependent excretion of scopoletin. Armillarisin A (3-acetyl-5-hydroxymethyl-7-hydroxycoumarin) is a coumarin derivative extracted from the fungus Armillariella tabescens (Scop. ex Fr.) Sing [55]. Chlorinated coumarins, 6-chloro-4-phenyl-2H-chromen-2-one and ethyl 6-chloro-2-oxo-4-phenyl-2H-chromen-3-carboxylate, were identified in the polypore mushroom Fomitopsis officinalis [56].
2.2. Classification of Naturally Occurring Coumarins and Their Role in Plant Protection
2.2.1. Simple Coumarins
Simple coumarins act in plants’ interaction with biotic and abiotic environmental stress factors. Secretion of iron-mobilizing coumarins by plant roots is a crucial factor for improving iron bioavailability in crops, enabling them to grow in iron-depleted soils. In Arabidopsis, three coumarins with iron-mobilizing properties are found: fraxetin (7,8-dihydroxy-6-methoxycoumarin), sideretin (5,7,8-trihydroxy-6-methoxycoumarin), and esculetin (6,7-dihydroxycoumarin) (Figure 2 Their catechol moiety (two neighboring hydroxyl groups) is thought to be a crucial structural feature for iron mobilization in soil [57]. Scopoletin (7-hydroxy-6-methoxy coumarin) (Figure 2) is a simple coumarin that occurs in Arabidopsis thaliana [58], and many other plants [59]. Physiologically, scopoletin protects against stress. Thus, it was proved that scopoletin accumulates in Arabidopsis leaves after the attack of the fungus Phakopsora pachyrhizi, which causes Asian soybean rust disease [60].
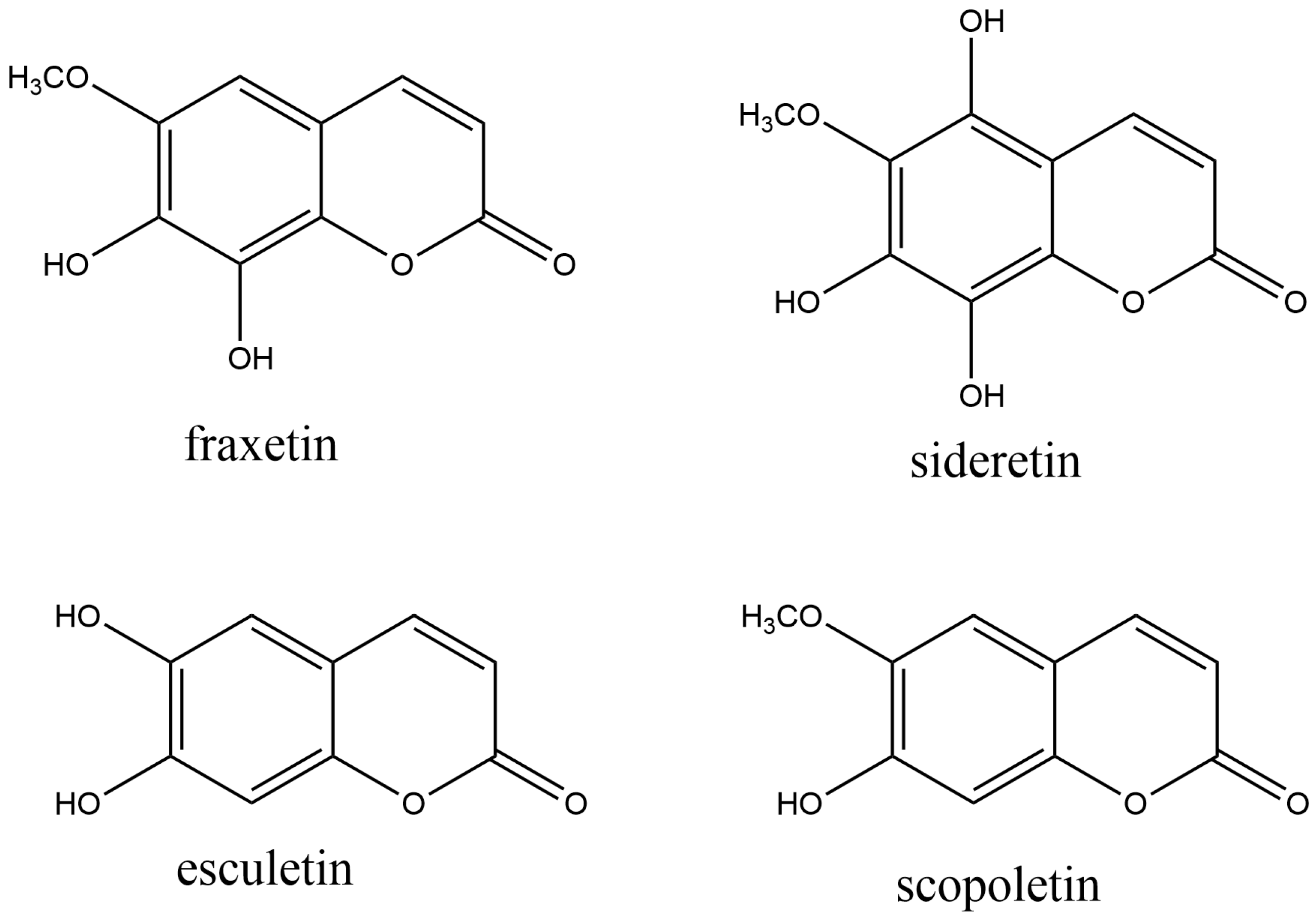
Figure 2. The most common naturally occurring simple coumarins.
In tobacco plants, scopoletin and its β-glucoside, scopolin, have physiological roles against stress, for example during tobacco mosaic virus infiltration [61]. Surangib B from Mammea longifolia inhibits mycelial growth of Rhizoctonia solani and Botrytis cinerea [62]. Umbelliferone (7-hydroxycoumarin) is a phytoalexin widely distributed within the Rutaceae and Apiaceae (Umbelliferae) families. It is an important phytoalexin that protects plants from pathogenic fungi, such as Fusarium culmorum [63], and the aerobic, Gram-negative, plant-pathogenic bacterium Ralstonia solanacearum [64].
Yang et al. [65] studied the antimicrobial activity of 18 natural compounds against Ralstonia solanacearum, the bacterium responsible for tobacco, tomatoes, and potatoes wilting in subtropical regions. The research showed that four coumarins, daphnetin, esculetin, umbelliferone, and xanthotol demonstrated stronger antibacterial effects than the standard treatment with thiadiazole and copper. A more detailed analysis showed that the enhanced antibacterial activity is due to the substitution at positions C-6, C-7, and C-8 of the coumarin nucleus. For this reason, they tested the activity of the hydroxycoumarins umbelliferone, esculetin, and daphnetin in the concentration range from 10 to 100 mg/L. Daphnetin (OH groups at positions C-7 and C-8) proved to be the most effective, esculetin (OH groups at positions C-6 and C-7) was somewhat weaker, while umbelliferone (OH group at positions C-7) showed the weakest activity. Thus, treatment of tobacco roots with umbelliferone prior to infection with R. solanacearum significantly reduced R. solanacearum biofilm formation, increasing resistance to disease [66]. Application of an elicitor of coumarin biosynthesis, salicylic acid to the roots of chamomile (Matricaria chamomilla) resulted in the accumulation of ambelliferone and herniarin (7-methoxy coumarin) in the leaves [67]. Herniarin suppressed R. solanacearum bacterial growth by destroying the bacterial cell membrane [68]. Also, plant-derived 6-methylcoumarin showed inhibitory effects against R. solanacearum, and suppressed tobacco bacterial wilt [69]. Derivatives of 3,4-dihydroisocoumarin isolated from endophytic fungus Lophiostoma sp., displayed antibacterial activities against Bacillus subtilis, Agrobacterium tumefaciens, R. solanacearum, and Xanthomonas vesicatoria [70].
Increased biosynthesis of coumarins, ayapin and scopoletin, has been observed in sunflowers (Helianthus annuus L.) during the attack of the sunflower beetle, Zygogramma exclamationis, which resulted in distracting further feeding of the beetle [71]. The coumarin (2H-1-benzopyran-2-one) proved effective against the green peach aphid Myzus persicae and friendly to the natural enemy of aphids, Harmonia axyridis and soil invertebrates, Eisenia fetida. The study implied that coumarin can be recommended as a selective and effective botanical aphicide friendly to non-target organisms. However, the environmental safety of a given insecticide must be estimated with further tests to clarify the mechanism of its action and efficacy [72]. Recently, five simple coumarin-based scaffolds (limetin-derivatives) were identified in Citrullus lanatus seeds, which possess significant bactericidal and fungicidal potential [73].
2.2.2. Furanocoumarins
Furanocoumarins are composed of a furan ring fused to a coumarin core (Figure 3).
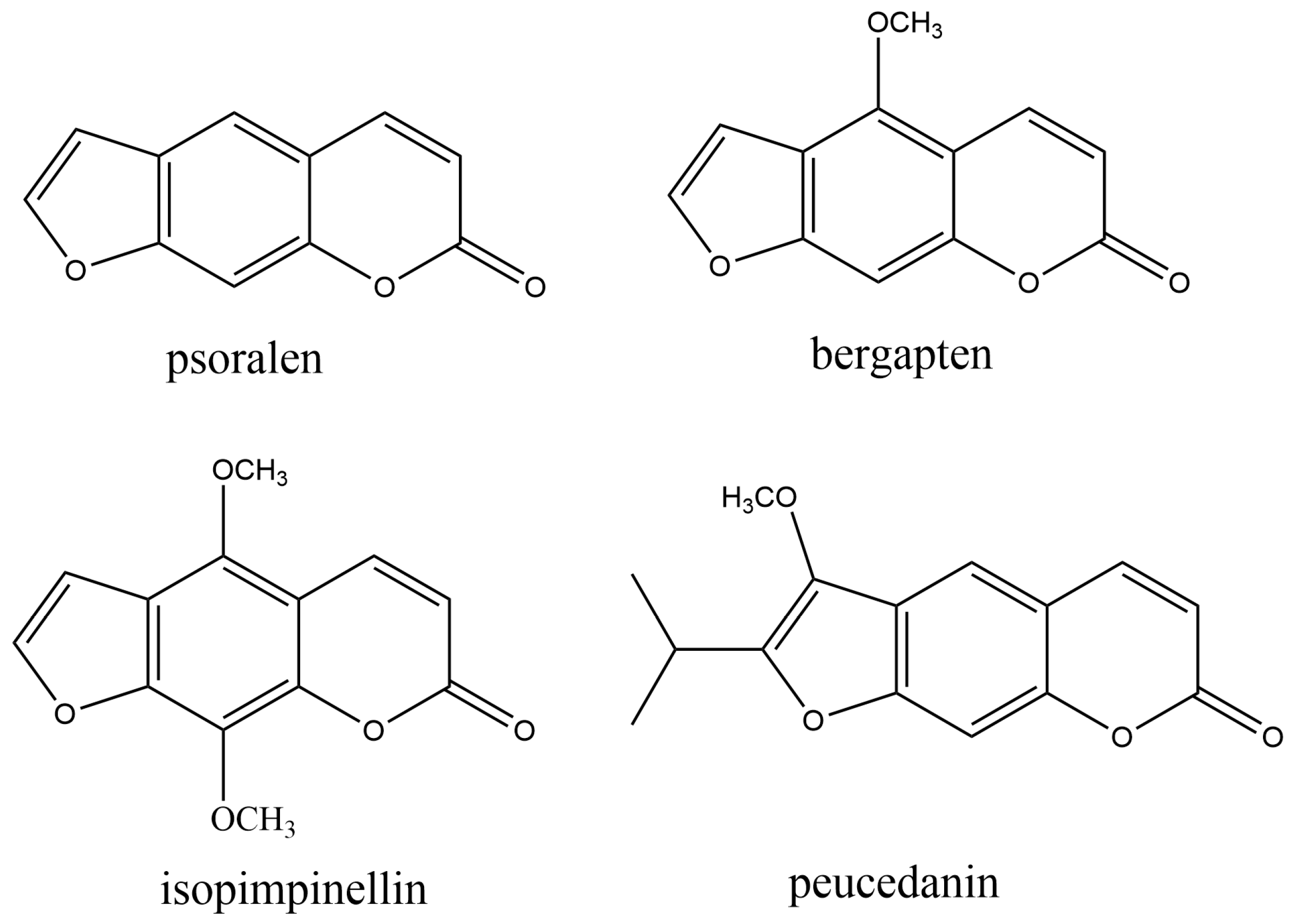
Figure 3. The most common naturally occurring furanocoumarins.
They are mostly present in plant families, including Apiaceae, Fabaceae, Moraceae, and Rutaceae. Their increased level in plants is a response to stress conditions, such as exposure to pathogenic fungi or to physical damage caused by occasional lesions or insect bites. They are involved in plant defense, acting against microorganisms, nematodes, phytophagous insects, herbivores, and plant competitors [74]. Linear furanocoumarins, such as psoralen, bergapten, isopimpinellin, and xanthotoxin, together with the angular dihydrofuranocoumarin athamantin, are antifeedants. Peucedanin (Figure 3) inhibits the growth of neonate larvae of Spodoptera litlis (Boisduval) (Lepidoptera: Noctuidae) [75]. Plants are a prominent source of novel nematicidal chemicals. Thus, furanocoumarins 8-geranyloxy psolaren, imperatorin, and heraclenin from the root extract of Heracleum candicans Wall., exhibited nematicidal effects against Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle and Pangrellus redivivus (Linn.) [76]. Also, bergapten and psoralen extract from Ficus carica L. leaves showed strong nematicidal activity against pine wood nematode (PWN), Bursaphelenchus xylophilus. Their nematicidal mechanism is probably based on the inhibition of amylase, cellulase, and acetylcholinesterase [77]. Ethanol extract from Chinese medicinal herb Notopterygium incisum rhizomes possessed strong nematicidal activity against two species of nematodes, Bursaphelenchus xylophilus and Meloidogyne incognita. The extract contained two furanocoumarins, colombienetin and isoimperatorin [78]. Essential oil and methanol extracts of parsley (Petroselinum crispum) [79], as well as ethanol extracts from Angelica pubescens Maxim. f. biserrata Shan et Yuan roots [80], exhibited promising nematicidal activity as a source of nematotoxic furanocoumarins.
A number of furanocoumarin compounds isolated from Semenovia transiliensis shoots have herbicidal activities [31]. Citrus plants produce simple coumarins and furanocoumarins to cope with herbivorous insects and pathogens [81]. Ramirez-Pelayo et al. [82] studied coumarin derivatives found in citrus peels. They isolated six coumarins (5-geranyloxy-7-methoxycoumarin, bergamottin, bergapten, isopimpineline, citropten, and oxypeucedanin hydrate) and tested their antifungal activity against Colletotrichum sp., a fungus causing fruit anthracnose. Their activity was compared with the simpler coumarins, umbelliferone, scoparone, and scopoletin. The test results showed that all six coumarins inhibited the growth of Colletotrichum sp. mycelia, and among them, bergapten and citropten proved to be the most effective. The research concluded that there is a synergistic effect between the individual coumarin components in the citrus peel extract.
2.2.3. Dihydrofuranocoumarins
The presence of dihydrofuranocoumarins in all plant parts, and especially the roots, is responsible for plants’ poisonous properties, such as Opopanax chironium (Apiaceae) [83] and Opopanax hispidus (Friv.) Griseb. [84]. It was found that dihydrofuranocoumarin xanthoarnol from the plant Xanthoxylum arnottianum (Rutaceae) showed an inhibitory effect on the germination of conidia of the parasitic fungus [85].
2.2.4. Phenylcoumarins
4-Phenylcoumarins protect plants against pests and fungi. Thus, 5,7-dimethoxy-4-p-methoxyphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin (Figure 4) were found in the microorganism Streptomyces aureofaciens, which was isolated from ginger root tissues (Zingiber officinale Rosc. (Zingiberaceae) were active against phytopathogenic fungi [86].

Figure 4. Naturally occurring 4-phenylcoumarins.
The naturally-occurring 3-phenylcoumarins were identified in plant species, mostly in the family Fabaceae, such as: mucodianin A from Mucuna birdwoodiana [87]; pterosonin A-F from heartwood of Pterocarpus soyauxii [88]; Sphenostylis marginata [50]; Pterocarpus soyauxii [89]; and selaginolide A from Vietnamese medicinal plant Selaginella rolandi-principis (Selaginellaceae) [90]. There is no evidence about their role in plants.
2.2.5. Pyranocoumarins
Pyranocoumarins are rare secondary metabolites of plants that contain a pyran core condensed with coumarin. These substances are distributed widely among the plant families Umbelliferae and Rutaceae. Although pyranocoumarins in the plant are very poorly studied, some studies indicate their protective role against phytopathogenic organisms [91]. However, pyranocoumarin isolated from the Rutaceae tree (Staurantus perforatus), xanthyletin, has shown significant phytotoxic effect on seed germination and root growth of Amarathus hypochondriacus (Amaranthaceae) [92]. Among the pyranocoumarins isolated from the roots of Ferulago campestris (Apiaceae), aegelinol and grandivittin have been shown to have cytotoxic properties [93]. Pyranocoumarin seselin isolated from Clausena anisata (Rutaceae) leaves acts as an antifeedant against Lucilia cuprina larvae [94].
2.2.6. Bicoumarines
Bicoumarines have been isolated from plants Triphasia trifolia (Rutaceae) [95], Dysoxylum parasiticum (Osbeck) Kosterm (Meliaceae) [96], and Pleurospermum rivulorum (Umbelliferae) [97]. The best-researched bicoumarin is dicoumarol (Figure 5). Dicumarol is generated by the hydroxylation of the 4-position of the coumarin. This is followed by capturing of a molecule of formaldehyde, and subsequently by condensation with another molecule of 4-hydroxycoumarin. Finally, the enolization of the keto group forms dicumarol [50]. Dicumarol is discovered as constituent of sweet clover hay that caused the death of cattle due to bleeding disorders. Dicoumarol is an anticoagulant that acts as a vitamin K antagonist [98]. The dicoumarol, also formed by bacterial fermentation of yellow sweet clover, was isolated for the first time from the decomposed leaves of Melilotus albus (Fabaceae/Leguminosae) [50].

Figure 5. Structure of naturally occurring bicoumarine—dicoumarol.
The presence of natural coumarins in different species of plants and microbes, and their biological effects are summarized in Table 1. It is evident that the diversity and structural complexity of the coumarins constitute is a consequence of higher plant evolution. Simple coumarins are the most common in fungi and all angiosperms. They exhibit a wide range of biological effects related to plant protection from pathogen microbes, fungi, and nematodes. Furanocoumarin is the second most prevalent type of coumarin. Furanocoumarins are found in the family Citrus, Apiaceae, and Rutaceae, with most of them showing nematicidal properties. Pyranocoumarins are present in Apiaceace and Fabaceae, where they exhibit antifungal, phytotoxic, and antifeedant effects. Phenylcoumarins are the most abundant in the plant family Fabaceae, but there is no literature on their functions in plants.
Table 1. The presence of natural coumarins in different species of microbes, spongi and plants with their biological effects.
| Phylum/ Famillies |
Species | Group of Coumarins | Specific Compounds | Known Biological Activities | Ref. |
|---|---|---|---|---|---|
| Bacteria | |||||
| Streptomyces | Streptomyces roseochromogenes var. oscitans | 3-amino-4,7-dihydroxycoumarins | clorobiocin, novobiocin, coumermycin |
antibacterial | [15] |
| Porifera | |||||
| Axinellidae | Axinella cf. corrugate | simple | esculetin-4-carboxylic acid esters | anti-SARS-CoV | [22] |
| Fungi | |||||
| Pleosporaceae | Alternaria alternata | simple | isofraxidin | antibacterial | [43] |
| Trichocomaceae | Aspergillus fumigatus Fresenius | simple, bicoumarins | 4-hydroxycoumarin, dicoumarol | biosynthesis of coumarin | [47][48] |
| Physalacriaceae | Armillariella tabescens | simple | armillarisin A | choleretic | [55] |
| Fomitopsidaceae | Fomitopsis officinalis | simple | 6-chloro-2-oxo-4-phenyl-coumarins | anti-TBC | [56] |
| Lophiostomataceae | Lophiostoma sp. Sigrf10 | 3,4-dihydroisocoumarin | lophiostomin derivatives | antifungal, antibacterial |
[70] |
| Plants | |||||
| Citrus | C. maxima, C. medica, C. reticulata, C. micrantha | simple, furanocoumarin | unknown | [81] | |
| Citrus sinensis, C. reticulata, C. aurantifolia | simple, furanocoumarin | limettin, isopimpinellin, psoralen, bergamottin | antifungal | [82] | |
| Cucurbitaceae | Citrullus lanatus | simple | derivates of 5,7-dimethoxycoumarin | antimicrobial | [73] |
| Apiaceae | Ferulago campestris | pyranocoumarin | aegelinol, grandivittin, | cytotoxicity | [93] |
| (or Umbelliferae) | furanocoumarin | bergapten, felamidin, isoimperatorin |
antimicrobial, antioxidant |
[16] | |
| Notopterygium incisum | dihidrofuranocoumarin | columbianetin | nematicidal | [78] | |
| linear furanocoumarin | isoimperatorin | nematicidal | |||
| Petroselinum crispum | furanocoumarins | xanthotoxin, psoralen, bergapten | nematicidal | [79] | |
| Angelica pubescens Maxim. f. biserrata Shan et Yuan | simple, dihidrofuranocoumarin, | osthole, columbianadin | nematicidal | [80] | |
| furanocoumarin | bergapten, xanthotoxin | nematicidal | [97] | ||
| Pleurospermum rivulorum | bicoumarin | rivulobirins | unknown | ||
| Opopanax hispidus(Friv.) Griseb. | dihydrofuranocoumarin | 3′-isobutyryl-3′-hydroxymarmesin | unknown | [84] | |
| simple, furanocoumarin | officinalin, oreoselon, peucedanin, | unknown | |||
| Peucedanum sp. | simple, furanocoumarin | ostruthin, osthol; isoimperatorin | insecticidal | [75] | |
| dihydropyranocoumarin | xanthalin, peuarenarin | insecticidal | |||
| dihydrofuranocoumarin | athamantin, columbianadin | insecticidal | |||
| Semenovia transiliensis | furanocoumarin | Imperatorin, xanthotoxin | herbicidal | [31] | |
| Heracleum candicans Wall. | uranocoumarin | 8-geranyloxy psolaren, imperatorin, heraclenin | nematicidal | [76] | |
| Fabaceae | Melilotus officinalis | simple | dihydrocoumarin | cytotoxicity | [24] |
| bicoumarin | dicoumarol | anticoagulant | [98] | ||
| Mucuna birdwoodiana | phenylcoumarin | mucodianin A | unknown | [89] | |
| Sphenostylis marginata | phenylcoumarin | sphenostylisin A | anticancer | [50] | |
| Pterocarpus soyauxii | phenylcoumarin | pterosonins | anticancer | [88] | |
| Millettia thonningii | pyranocoumarin, furanocoumarin |
robustic acid, thonningine-C | antifungal | [20] | |
| Solanaceae | Nicotiana tabacum | simple | scopolin, scopoletin | antiviral | [61] |
| Lamiaceae | Baikal skullcap | simple | 7.8-dihydroxy-4-methylcumarin | antibacterial | [17] |
| Brassicales | Arabidopsis thaliana | simple | scopoletin | antifungal | [60] |
| Moraceae | Ficus carica | furocoumarin | bergapten, psoralen | nematicidal | [77] |
| Meliaceae | Dysoxylum parasiticum (Osbeck) Kosterm | bicoumarin | bidysoxyletine | unknown | [96] |
| Rutaceae | Triphasia trifolia | simple, furocoumarin | umbelliferone, isopimpinellin, | unknown | [95] |
| Xanthoxylum arnottianum | dihydrofuranocoumarin | xanthoarnol | antifungal | [85] | |
| Staurantus perforatus | pyranocoumarin | xanthyletin | phytotoxic | [92] | |
| Ruta angustifolia | furocoumarin, dihydrofuranocoumarin |
chalepensin, chalepin | anticancer, antiviral |
[28] | |
| Clausena anisata | pyranocoumarin | seselin | antifeedant | [94] | |
| Thymelaeaceae | Wikstroemia indica (L.) | bicoumarin | daphnoretin | antiviral, antitumor | [29] |
| Calophyllaceae | Mammea longifolia | simple | surangib B | antifungal | [62] |
This entry is adapted from the peer-reviewed paper 10.3390/app13116535
References
- Sánchez-Bayo, F.; Tennekes, H.A. Environmental Risk Assessment of Agrochemicals—A Critical Appraisal of Current Approaches. In Toxicity and Hazard of Agrochemicals; Larramendy, M.L., Soloneski, S., Eds.; InTech: London, UK, 2015.
- Li, Z. A disease-specific screening-level modeling approach for assessing the cancer risks of pesticide mixtures. Chemosphere 2022, 286, 131811.
- Li, Z. Prioritizing agricultural pesticides to protect human health: A multi-level strategy combining life cycle impact and risk assessments. Ecotoxicol. Environ. Saf. 2022, 242, 113869.
- Polyxeni, N.-S.; Sotirios, M.; Chrysanthi, K.; Panagiotis, S.; Luc, H. Pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148.
- Smith, K.; Evans, D.A.; El-Hiti, G.A. Role of modern chemistry in sustainable arable crop protection. Philos. Trans. R. Soc. B 2008, 363, 623–637.
- European Parliament. Directive 2009/128/EC of the European Parliament and of the Council of Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides. 21 October 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009L0128 (accessed on 4 November 2022).
- Hussain, M.I.; Qamar Abbas, S.; Reigosa, M.J. Activities and novel applications of secondary metabolite coumarins. Planta Daninha 2017, 35, e017174040.
- Kadhum, A.A.H.; Al-Amiery, A.A.; Musa, A.Y.; Mohamad, A.B. The antioxidant activity of new coumarin derivatives. Int. J. Mol. Sci. 2011, 12, 5747–5761.
- Molnar, M.; Komar, M.; Brahmbhatt, H.; Babić, J.; Jokić, S.; Rastija, V. Deep eutectic solvents as convenient media for synthesis of novel coumarinyl schiff bases and their QSAR studies. Molecules 2017, 22, 1482.
- Al-Majedy, Y.K.; Al-Duhaidahawi, D.L.; Al-Azawi, K.F.; Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.B. Coumarins as potential antioxidant agents complemented with suggested mechanisms and approved by molecular modeling studies. Molecules 2016, 21, 135.
- Sahoo, C.R.; Sahoo, J.; Mahapatra, M.; Lenka, D.; Sahu, P.K.; Dehury, B.; Padhy, R.N.; Paidesetty, S.K. Coumarin derivatives as promising antibacterial agent(s). Arab. J. Chem. 2021, 14, 102922.
- Bhagat, K.; Bhagat, J.; Gupta, M.K.; Singh, J.V.; Gulati, H.K.; Singh, A.; Kaur, K.; Kaur, G.; Sharma, S.; Rana, A.; et al. Design, synthesis, antimicrobial evaluation, and molecular modeling studies of novel indolinedione–coumarin molecular hybrids. ACS Omega 2019, 4, 8720–8730.
- Cheke, R.S.; Patel, H.M.; Patil, V.M.; Ansari, I.A.; Ambhore, J.P.; Shinde, S.D.; Kadri, A.; Snoussi, M.; Adnan, M.; Kharkar, P.S.; et al. Molecular insights into coumarin analogues as antimicrobial agents: Recent developments in drug discovery. Antibiotics 2022, 11, 566.
- Završnik, D.; Špirtović-Halilović, S.; Softić, D. Synthesis, structure and antibacterial activity of 3-substituted derivatives of 4-hydroxycoumarin. Period. Biol. 2011, 133, 93–97. Available online: https://hrcak.srce.hr/67271 (accessed on 2 January 2023).
- Eustáquio, A.S.; Gust, B.; Luft, T.; Li, S.-M.; Chater, K.F.; Heide, L. Clorobiocin biosynthesis in Streptomyces: Identification of the halogenase and generation of structural analogs. Chem. Biol. 2003, 10, 279–288.
- Süzgeç-Selçuk, S.; Dikpınar, T. Phytochemical evaluation of the Ferulago genus and the pharmacological activities of its coumarin constituents. J. Herb. Med. 2021, 21, 100415.
- Deryabin, D.; Inchagova, K.; Rusakova, E.; Duskaev, G. Coumarin’s anti-quorum sensing activity can be enhanced when combined with other plant-derived small molecules. Molecules 2021, 26, 208.
- De Araújo, R.S.A.; Guerra, F.Q.S.; de O Lima, E.; De Simone, C.A.; Tavares, J.F.; Scotti, L.; Scotti, M.T.; De Aquino, T.M.; De Moura, R.O.; Mendonça, F.J.B.; et al. Synthesis, structure-activity relationships (SAR) and in silico studies of coumarin derivatives with antifungal activity. Int. J. Mol. Sci. 2013, 14, 1293–1309.
- Lemos, A.S.O.; Florêncio, J.R.; Pinto, N.C.C.; Campos, L.M.; Silva, T.P.; Grazul, R.M.; Pinto, P.F.; Tavares, G.D.; Scio, E.; Apolônio, A.C.M.; et al. Antifungal activity of the natural coumarin scopoletin against planktonic cells and biofilms from a multidrug-resistant Candida tropicalis strain. Front. Microbiol. 2020, 11, 1525.
- Ayine-Tora, D.M.; Kingsford-Adaboh, R.; Asomaning, W.A.; Harrison, J.J.E.K.; Mills-Robertson, F.C.; Bukari, Y.; Sakyi, P.O.; Kaminta, S.; Reynisson, J. Coumarin Antifungal lead compounds from Millettia thonningii and their predicted mechanism of action. Molecules 2016, 21, 1369.
- Xu, Z.; Chen, Q.; Zhang, Y.; Liang, C. Coumarin-based derivatives with potential anti-HIV activity. Fitoterapia 2021, 150, 104863.
- Hamoda, A.M.; Fayed, B.; Ashmawy, N.S.; El-Shorbagi, A.A.; Hamdy, R.; Soliman, S.S.M. Marine sponge is a promising natural source of anti-SARS-CoV-2 scaffold. Front. Pharmacol. 2021, 12, 666664.
- Hu, Y.-Q.; Xu, Z.; Zhang, S.; Wu, X.; Ding, J.-W.; Lv, Z.-S.; Feng, L.-S. Recent developments of coumarin-containing derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017, 136, 122–130.
- Olaharski, A.J.; Rine, J.; Marshall, B.L.; Babiarz, J.; Zhang, L.; Verdin, E.; Smith, M.T. The flavoring agent dihydrocoumarin reverses epigenetic silencing and inhibits sirtuin deacetylases. PLoS Genet. 2005, 1, e77.
- Mohamed, T.K.; Batran, R.Z.; Elseginy, S.A.; Ali, M.M.; Mahmoud, A.E. Synthesis, anticancer effect and molecular modeling of new thiazolylpyrazolyl coumarin derivatives targeting VEGFR-2 kinase and inducing cell cycle arrest and apoptosis. Bioorg. Chem. 2019, 85, 253–273.
- Wu, X.-Q.; Huang, C.; Jia, Y.-M.; Song, B.-A.; Li, J.; Liu, X.-H. Novel coumarin-dihydropyrazole thio-ethanone derivatives: Design, synthesis and anticancer activity. Eur. J. Med. Chem. 2014, 74, 717–725.
- Lu, W.; Tang, J.; Gu, Z.; Sun, L.; Wei, H.; Wang, Y.; Yang, S.; Chi, X.; Xu, L. Crystal structure, in vitro cytotoxicity, DNA binding and DFT calculations of new copper (II) complexes with coumarin-amide ligand. J. Inorg. Biochem. 2023, 238, 112030.
- Bailly, C. Ruta angustifolia Pers. (Narrow-Leaved Fringed Rue): Pharmacological Properties and Phytochemical Profile. Plants 2023, 12, 827.
- Huang, Y.-C.; Huang, C.-P.; Lin, C.-P.; Yang, K.-C.; Lei, Y.-J.; Wang, H.-P.; Kuo, Y.-H.; Chen, Y.-J. Naturally occurring bicoumarin compound daphnoretin inhibits growth and induces megakaryocytic differentiation in human chronic myeloid leukemia cells. Cells 2022, 11, 3252.
- Razavi, S.M. Plants coumarins as allelopathic agents. Int. J. Org. Chem. 2011, 5, 86–90. Available online: https://scialert.net/abstract/?doi=ijbc.2011.86.90 (accessed on 2 January 2023).
- Sondhia, S.; Duke, S.O.; Green, S.; Gemejiyeva, N.G.; Mamonov, L.K.; Cantrell, C.L. Phytotoxic furanocoumarins from the shoots of Semenovia transiliensis. Nat. Prod. Commun. 2012, 7, 1327–1330.
- Song, P.P.; Zhao, J.; Liu, Z.-L.; Duan, Y.B.; Hu, Y.-P.; Zhao, C.-Q.; Wu, M.; Wei, M.; Wang, N.-H.; Lv, Y.; et al. Evaluation of antifungal activities and structure–activity relationships of coumarin derivatives. Pest Manag. Sci. 2017, 73, 94–101.
- De Andrade Gonçalves, P.; dos Santos Junior, M.C.; do Sacramento Sousa, C.; Góes-Neto, A.; Luz, E.D.M.N.; Damaceno, V.O.; Niella, A.R.R.; Filho, J.M.B.; de Assis, S. A Study of sodium 3-hydroxycoumarin as inhibitors in vitro, in vivo and in silico of Moniliophthora perniciosa fungus. Eur. J. Plant Pathol. 2019, 153, 15–27.
- Wei, Y.; Peng, W.; Wanf, D.; Hao, S.-H.; Li, W.-W.; Ding, F. Design, synthesis, antifungal activity, and 3D-QSAR of coumarin derivatives. J. Pestic. Sci. 2018, 43, 88–95.
- Rastija, V.; Vrandečić, K.; Ćosić, J.; Majić, I.; Kanižai Šarić, G.; Agić, D.; Karnaš, M.; Lončarić, M.; Molnar, M. Biological activities related to plant protection and environmental effects of coumarin derivatives: QSAR and molecular docking studies. Int. J. Mol. Sci. 2021, 22, 7283.
- Rastija, V.; Vrandečić, K.; Ćosić, J.; Šarić, G.K.; Majić, I.; Agić, D.; Šubarić, D.; Karnaš, M.; Bešlo, D.; Komar, M.; et al. Effects of coumarinyl Schiff bases against phytopathogenic fungi, the soil-beneficial bacteria and entomopathogenic nematodes: Deeper insight into the mechanism of action. Molecules 2022, 27, 2196.
- Chen, J.; Yu, Y.; Li, S.; Ding, W. Resveratrol and coumarin: Novel agricultural antibacterial agent against Ralstonia solanacearum in vitro and in vivo. Molecules 2016, 21, 1501.
- Rehman, S.; Ikram, M.; Baker, R.J.; Zubair, M.; Azad, E.; Min, S.; Riaz, K.; Mok, K.H.; Rehman, S.-U. Synthesis, characterization, in vitro antimicrobial, and U2OS tumoricidal activities of different coumarin derivatives. Chem. Cent. J. 2013, 7, 68. Available online: http://hdl.handle.net/2262/69349 (accessed on 15 February 2023).
- Dekić, B.R.; Radulović, N.S.; Dekić, V.S.; Vukićević, R.D.; Palić, R.M. Synthesis and antimicrobial activity of new 4-heteroarylamino coumarin derivatives containing nitrogen and sulfur as heteroatoms. Molecules 2010, 15, 2246–2256.
- Cui, H.; Jin, H.; Liu, Q.; Yan, Z.; Ding, L.; Qin, B. Nematicidal metabolites from roots of Stellera chamaejasme against Bursaphelenchus xylophilus and Bursaphelenchus mucronatus. Pest Manag. Sci. 2014, 70, 827–835.
- Pan, L.; Li, X.-Z.; Sun, S.-A.; Guo, H.-R.; Qin, B. Design and synthesis of novel coumarin analogs and their nematicidal activity against five phytonematodes. Chin. Chem. Lett. 2016, 27, 375–379.
- Stringlis, I.A.; de Jonge, R.; Pieterse, C.M.J. The age of coumarins in plant–microbe interactions. Plant Cell Physiol. 2019, 60, 1405–1419.
- Srinivasa, C.; Mellappa, G.; Patil, S.M.; Ramu, R.; Shreevatsa, B.; Dharmashekar, C.; Kollur, S.P.; Syed, A.; Shivamallu, C. Plants and endophytes—A partnership for the coumarin production through the microbial systems. Mycology 2022, 13, 243–256.
- Perkowska, I.; Potrykus, M.; Siwinska, J.; Siudem, D.; Lojkowska, E.; Ihnatowicz, A. Interplay between coumarin accumulation, iron deficiency and plant resistance to Dickeya spp. Int. J. Mol. Sci. 2021, 22, 6449.
- Yang, Y.; Xu, J.; Li, Y.; He, Y.; Yang, Y.; Liu, D.; Wu, C. Effects of coumarin on rhizosphere microbiome and metabolome of Lolium multiflorum. Plants 2023, 12, 1096.
- Harbort, C.J.; Hashimoto, M.H.; Inoue, H.; Niu, Y.; Guan, R.; Rombolá, A.D.; Kopriva, S.; Voges, M.J.; Sattely, E.S.; Garrido-Oter, R.; et al. Root-secreted coumarins and the microbiota interact to improve iron nutrition in Arabidopsis. Cell Host Microbe 2020, 28, 825–837.
- Bye, A.; King, H.K. The biosynthesis of 4-hydroxycoumarin and dicoumarol by Aspergillus fumigatus Fresenius. Biochem. J. 1970, 117, 237–245.
- Aguirre-Pranzoni, C.; Orden, A.A.; Bisogno, F.R.; Ardanaz, C.E.; Tonn, C.E.; Kurina-Sanz, M. Coumarin metabolic routes in Aspergillus spp. Fungal Biol. 2011, 115, 245–252.
- Hoult, J.; Paya, M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmac. 1996, 27, 713–722.
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An important class of phytochemicals. In Phytochemicals: Isolation, Characterisation and Role in Human Health; Rao, A.V., Rao, L.G., Eds.; InTech: London, UK, 2015.
- Shimizu, B.-I. 2-Oxoglutarate-dependent dioxygenases in the biosynthesis of simple coumarins. Front. Plant Sci. 2014, 5, 549.
- Wu, F.; Duan, Z.; Xu, P.; Yan, Q.; Meng, M.; Cao, M.; Jones, C.S.; Zong, X.; Zhou, P.; Wang, Y.; et al. Genome and systems biology of Melilotus albus provides insights into coumarins biosynthesis. Plant Biotechnol. J. 2022, 20, 592–609.
- Vismans, G.; van Bentum, S.; Spooren, J.; Song, Y.; Goossens, P.; Valls, J.; Snoek, B.L.; Thiombiano, B.; Schilder, M.; Dong, L.; et al. Coumarin biosynthesis genes are required after foliar pathogen infection for the creation of a microbial soil-borne legacy that primes plants for SA-dependent defenses. Sci. Rep. 2022, 12, 22473.
- Stringlis, I.A.; Yu, K.; Feussner, K.; de Jonge, R.; Van Bentum, S.; Van Verk, M.C.; Berendsen, R.L.; Bakker, P.A.; Feussner, I.; Pieterse, C.M. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl. Acad. Sci. USA 2018, 115, E5213–E5222.
- Wang, Y.; Wang, Y.; Li, P.; Tang, Y.; Fawcett, J.P.; Gu, J. Quantitation of Armillarisin A in human plasma by liquid chromatography–electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007, 43, 1860–1863.
- Hwang, C.H.; Jaki, B.U.; Klein, L.L.; Lankin, D.C.; McAlpine, J.B.; Napolitano, J.G.; Fryling, N.A.; Franzblau, S.G.; Cho, S.H.; Stamets, P.E.; et al. Cholinated coumarins from the polypore mushroom Fomitopsis officialis and their activity against Mycobacterium tuberculosis. J. Nat. Prod. 2013, 76, 1916–1922.
- Robe, K.; Izquierdo, E.; Vignols, F.; Rouached, H.; Dubos, C. The coumarins: Secondary metabolites playing a primary role in plant nutrition and health. Trends Plant Sci. 2021, 26, 248–259.
- Kai, K.; Shimizu, B.; Mizutani, M.; Watanabe, K.; Sakata, K. Accumulation of coumarins in Arabidopsis thaliana. Phytochemistry 2006, 67, 379–386.
- Gnonlonfin, G.J.B.; Sanni, A.; Brimer, L. Review Scopoletin—A coumarin phytoalexin with medicinal properties. Crit. Rev. Plant Sci. 2012, 31, 47–56.
- Beyer, S.F.; Beesley, A.; Rohmann, P.F.W.; Schultheiss, H.; Conrath, U.; Langenbach, J.G. The Arabidopsis non-host defence-associated coumarin scopoletin protects soybean from Asian soybean rust. Plant J. 2019, 99, 397–413.
- Chong, J.; Baltz, R.; Schmitt, C.; Beffa, R.; Fritig, B.; Saindrenan, P. Downregulation of a pathogen-responsive tobacco UDPGlc: Phenylpropanoid glucosyltransferase reduces scopoletin glucoside accumulation, enhances oxidative stress, and weakens virus resistance. Plant Cell 2002, 14, 1093–1107.
- Deng, Y.; Nicholson, R.A. Antifungal properties of surangin B, a coumarin from Mammea longifolia. Planta Med. 2005, 71, 364–365.
- Mazimba, O. Umbelliferone: Sources, chemistry and bioactivities review. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 223–232.
- Yang, L.; Wu, L.; Yao, W.; Zhao, S.; Wang, J.; Li, S.; Ding, W. Hydroxycoumarins: New, effective plant-derived compounds reduce Ralstonia pseudosolanacearum populations and control tobacco bacterial wilt. Microbiol. Res. 2018, 215, 15–21.
- Yang, L.; Ding, W.; Xu, Y.; Wu, D.; Li, S.; Chen, J.; Guo, B. New insights into the antibacterial activity of hydroxycoumarins against Ralstonia solanacearum. Molecules 2016, 21, 468.
- Yang, L.; Li, S.; Qin, X.; Jiang, G.; Chen, J.; Li, B.; Yao, X.; Liang, P.; Zhang, Y.; Ding, W. Exposure to umbelliferone reduces Ralstonia solanacearum biofilm formation, transcription of type III secretion system regulators and effectors and virulence on tobacco. Front. Microbiol. 2017, 8, 1234.
- Pastirova, A.; Repcak, M.; Eliasova, A. Salicylic acid induces changes of coumarin metabolites in Matricaria chamomilla L. Plant Sci. 2004, 167, 819–824.
- Han, S.; Yang, L.; Wang, Y.; Ran, Y.; Li, S.; Ding, W. Preliminary studies on the antibacterial mechanism of a new plant-derived compound, 7-methoxycoumarin, against Ralstonia solanacearum. Front. Microbiol. 2021, 12, 697911.
- Yang, L.; Wang, Y.; He, X.; Xiao, Q.; Han, S.; Jia, Z.; Li, S.; Ding, W. Discovery of a novel plant-derived agent against Ralstonia solanacearum by targeting the bacterial division protein FtsZ. Pestic. Biochem. Physiol. 2021, 177, 104892.
- Mao, Z.; Xue, M.; Gu, G.; Wang, W.; Li, D.; Lai, D.; Zhou, L. Synthesis and antibacterial activity of novel chalcone derivatives bearing a coumarin moiety. Lophiostomin A–D: New 3,4-dihydroisocoumarin derivatives from the endophytic fungus Lophiostoma sp. Sigrf10. RSC Adv. 2020, 10, 6985.
- Olson, M.M.; Roseland, C.R. Induction of the coumarins scopoletin and ayapin in sunflower by insect–feeding stress and effects of coumarins on the feeding of sunflower beetle (Coleoptera: Chrysomelidae). Environ. Entomol. 1991, 20, 1166–1172.
- Pavela, R.; Maggi, F.; Benelli, G. Coumarin (2H-1-benzopyran-2-one): A novel and eco-friendly aphicide. Nat. Prod. Res. 2019, 35, 1566–1571.
- Jebir, R.M.; Mustafa, Y.F. Novel coumarins isolated from the seeds of Citrullus lanatus as potential antimicrobial agents. Eurasian Chem. Commun. 2022, 4, 692–708.
- Bruni, R.; Barreca, D.; Protti, M.; Brighenti, V.; Righetti, L.; Anceschi, L.; Mercolini, L.; Benvenuti, S.; Gattuso, G.; Pellati, F. Botanical sources, chemistry, analysis, and biological activity of furanocoumarins of pharmaceutical interest. Molecules 2019, 24, 2163.
- Hadaček, F.; Müller, C.; Werner, A.; Greger, H.; Proksch, P. Analysis, isolation and insecticidal activity of linear furanocoumarins and other coumarin derivatives from Peucedanum (Apiaceae: Apioideae). J. Chem. Ecol. 1994, 20, 2035–2054.
- Wang, X.-B.; Li, G.-H.; Li, L.; Zheng, L.-J.; Huang, R.; Zhang, K.-Q. Nematicidal coumarins from Heracleum candicans Wall. Nat. Prod. Res. 2008, 22, 666–671.
- Guo, Q.; Du, G.; He, H.; Xu, H.; Guo, D.; Li, R. Two nematicidal furocoumarins from Ficus carica L. leaves and their physiological effects on pine wood nematode (Bursaphelenchus xylophilus). Nat. Prod. Res. 2016, 30, 1969–1973.
- Liu, G.; Lai, D.; Liu, Q.Z.; Zhou, L.; Liu, Z.L. Identification of nematicidal constituents of Notopterygium incisum rhizomes against Bursaphelenchus xylophilus and Meloidogyne incognita. Molecules 2016, 21, 1276.
- Caboni, P.; Saba, M.; Oplos, C.; Aissani, N.; Maxia, A.; Menkissoglu-Spiroudi, U.; Casua, L.; Ntallia, N. Nematicidal activity of furanocoumarins from parsley against Meloidogyne spp. Pest Manag. Sci. 2015, 71, 1099–1105.
- Guo, Q.-Q.; Du, G.-C.; Li, Y.-X.; Liang, C.-Y.; Wang, C.; Zhang, Y.-N.; Li, R.-G. Nematotoxic coumarins from Angelica pubescens Maxim. f. biserrata Shan et Yuan roots and their physiological effects on Bursaphelenchus xylophilus. J. Nematol. 2018, 50, 1–10.
- Dugrand-Judek, A.; Olry, A.; Hehn, A.; Costantino, G.; Ollitrault, P.; Froelicher, Y.; Bourgaud, F. The distribution of coumarins and furanocoumarins in Citrus species closely matches citrus phylogeny and reflects the organization of biosynthetic pathways. PLoS ONE 2015, 10, e0142757.
- Ramírez-Pelayo, C.; Martínez-Quiñones, J.; Gil, J.; Durango, D. Coumarins from the peel of citrus grown in Colombia: Composition, elicitation and antifungal activity. Heliyon 2019, 5, e01937.
- Appendino, G.; Bianchi, F.; Bader, A.; Campagnuolo, C.; Fattorusso, E.; Taglialatela-Scafati, O.; Blanco-Molina, M.; Macho, A.; Fiebich, B.L.; Bremner, P.; et al. Coumarins from Opopanax chironium. New dihydrofuranocoumarins and differential induction of apoptosis by imperatorin and heraclenin. J. Nat. Prod. 2004, 67, 532–536.
- Ghasemi, S.; Habibi, Z. A new dihydrofuranocoumarin from Opopanax hispidus (Friv.) Griseb. Nat. Prod. Res. 2014, 28, 1808–1812.
- Alami, I.; Clerivet, A.; Naji, M.; Munster, M.V.; Macheix, J.J. Elicitation of Platanus_acerifolia cell-suspension cultures induces the synthesis of xanthoarnol, a dihydrofuranocoumarin phytoalexin. Phytochemistry 1999, 51, 733–736.
- Taechowisan, T.; Lu, C.; Shen, Y.; Lumyong, S. Secondary metabolites from endophytic Streptomyces aureofaciens CMUAc130 and their antifungal activity. Microbiology 2005, 151, 1691–1695.
- Gong, T.; Wang, D.X.; Yang, Y.; Liu, P.; Chen, R.Y.; Yu, D.Q. A novel 3-arylcoumarin and three new 2-arylbenzofurans from Mucuna birdwoodiana. Chem. Pharm. Bull. 2010, 58, 254–256.
- Su, Z.; Wang, P.; Yuan, W.; Li, S. Flavonoids and 3-arylcoumarin from Pterocarpus soyauxii. Planta Med. 2013, 79, 487–491.
- Matos, M.J.; Uriarte, E.; Santana, L. 3-Phenylcoumarins as a privileged scaffold in medicinal chemistry: The Landmarks of the past decade. Molecules 2021, 26, 6755.
- Nguyen, D.T.; To, D.C.; Tran, T.T.; Tran, M.H.; Nguyen, P.H. PTP1B and-glucosidase inhibitors from Selaginella rolandi-principis and their glucose uptake stimulation. J. Nat. Med. 2021, 75, 186–193.
- Khandy, M.T.; Sofronova, A.K.; Gorpenchenko, T.Y.; Chirikova, N.K. Plant pyranocoumarins: Description, biosynthesis, application. Plants 2022, 11, 3135.
- Anaya, A.L.; Macías-Rubalcava, M.; Cruz-Ortega, R.; García-Santana, C.; Sánchez-Monterrubio, P.N.; Hernández-Bautista, B.E.; Mata, R. Allelochemicals from Stauranthus perforatus, a Rutaceous tree of the Yucatan Peninsula, Mexico. Phytochemistry 2005, 66, 487–494.
- Rosselli, S.; Maggio, A.M.; Faraone, N.; Spadaro, V.; Morris-Natschke, S.L.; Bastow, K.F.; Lee, K.-H.; Bruno, M. The cytotoxic properties of natural coumarins isolated from roots of Ferulago campestris (Apiaceae) and of synthetic ester derivatives of aegelinol. Nat. Prod. Commun. 2009, 4, 1701–1706.
- Mukandiwa, L.; Ahmed, A.; Eloff, J.N.; Naidoo, V. Isolation of seselin from Clausena anisata (Rutaceae) leaves and its effects on the feeding and development of Lucilia cuprina larvae may explain its use in ethnoveterinary medicine. J. Ethnopharmacol. 2013, 2, 886–891.
- Dondon, R.; Bourgeois, P.; Fery-Forgues, S. A new bicoumarin from the leaves and stems of Triphasia trifolia. Fitoterapia 2006, 77, 129–133.
- Sofian, F.F.; Subarnas, A.; Koseki, T.; Shiono, Y. Structure elucidation of a new bicoumarin derivative from the leaves of Dysoxylum parasiticum (Osbeck) Kosterm. Magn. Reson. Chem. 2022, 60, 857–863.
- Xiao, Y.-Q.; Liu, X.-H.; Taniguchi, M.; Baba, K. Bicoumarins from Pleurosperum rivulorum. Phytochemistry 1997, 45, 1275–1277.
- Timson, D.J. Dicoumarol: A drug which hits at least two very different targets in vitamin K metabolism. Curr. Drug Targets 2017, 18, 500–510.
This entry is offline, you can click here to edit this entry!
