Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Cigarette smoking has a significant impact on public health. In addition to the well-known role in several cancers, and for this reason identified as a group one carcinogen by the IARC classification, it has been linked to an increased disability rate and to several chronic conditions as cardiovascular, pneumological, endocrine, periodontal, or musculoskeletal diseases and, more in general, to 10 years reduced life expectancy.
- lung cancer
- genitourinary cancer
- environmental exposure
1. Introduction
According to the World Health Organization (WHO) estimates in 2019, cancer is one of the leading causes of death in almost 112 out of 183 countries [1]. In most cases, cancer etiology is multifactorial: different elements, as well as genetic predisposition or exposure to environmental agents, can play a crucial role [2]. Risk factors such as age, sex, and family history are actually intrinsic and not modifiable, meanwhile, others, such as exposure to environmental or occupational agents or lifestyle (i.e., tobacco habit, obesity, diet, and alcohol consumption) can be partially or totally adjusted [3,4].
Some agents, such as tobacco smoking, polycyclic aromatic hydrocarbons (PAHs), and arsenic, may predispose simultaneously to different neoplasms, in particular, lung (LC) and genitourinary (GUC) cancers (Figure 1 and Figure 2). These are the neoplasms with the highest incidence and mortality worldwide. More particularly, LC is the second most diagnosed cancer and the leading cause of cancer related deaths with an estimated 1.8 million deaths in 2020 [1,5]. LC encompasses different histopathology subtypes, historically classified into small cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC). Among NSCLCs, adenocarcinoma is the most common subtype (40%), followed by squamous cell or adenosquamous carcinoma and large cell neuroendocrine carcinoma. Tobacco smoking is the most relevant risk factor for LC occurrence, being responsible of two-thirds of all cases of LC [6]. Other risk factors comprise environmental air pollution or exposure to different agents such as asbestos, radon, cadmium, PAHs, diesel exhaust, and household smoke.
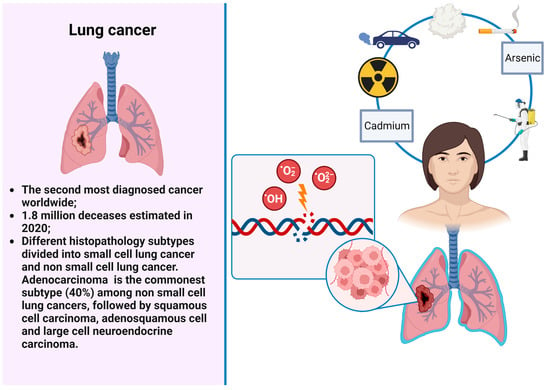
Figure 1. LC and environmental risks factors.
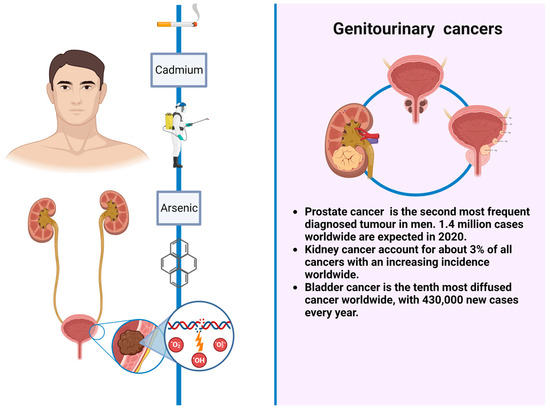
Figure 2. GUCs and environmental risks factors.
In regard to GUCs, prostate cancer is the second most frequent diagnosed tumor in men, with an estimated 1.4 million cases worldwide in 2020 [7]. Different risk factors have been taken into account in prostate cancer occurrence, such as arsenic, cadmium, or pesticides exposure. Renal cell carcinoma represents only 3% of all cancers, with the highest incidence occurring in Western countries. Established risk factors include cigarette smoking, obesity, and hypertension. In the last two decades, renal cell carcinoma incidence has been increasing by approximately 2–4% per year both for a higher rate of incidental tumors and probably for exposure to environmental and occupational cancerogenic factors that nowadays are only partially known [8]. Urothelial neoplasms comprise upper tract urothelial cancers such as renal pelvis and ureter neoplasms and lower tract tumors as bladder cancers; these are the most frequent in this category, being the ninth most diffused, most common cancer worldwide, with 430,000 new cases every year. Tobacco smoking is the most well-established risk factor for bladder cancer, accounting for 50–65% and 20–30% of cases among men and women, respectively [9]. Other risk factors include aromatic amines, PAHs, arsenic, and diesel exhaust exposure [10,11,12,13]. Upper tract urothelial cancers share almost the same risk factors of bladder cancers and, despite the common histological origin, behave differently, being more aggressive and frequently diagnosed at an advanced stage [10,11,12,13,14].
2. Tobacco Smoking
Cigarette smoking has a significant impact on public health. In addition to the aforementioned role in several cancers, being otherwise classified in the group one carcinogens by the IARC, it has been linked to an increased disability rate and to several chronic conditions as cardiovascular, pneumological, endocrine, periodontal, or musculoskeletal diseases and, more in general, to 10 years reduced life expectancy [16].
Up to date, almost 60 different tobacco-smoking-related compounds have been taken into account in playing a role in tumorigenesis [17]. Among these, nitrosamine such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N’-nitrosonornicotine (NNN), polycyclic aromatic hydrocarbons, arylamines, particulate matter, and volatile organics should be mentioned, due to their impactful role in cancer occurrence [18]. In this regard, for example, PAHs interacting with nucleic acids lead to DNA adducts in specific genes such as p53 and KRAS, both correlated to cancer onset. PAHs are also implicated in reactive oxygen species (ROS) synthesis, highly related to cell inflammation and, again, with cancer occurrence. Similar mechanisms are otherwise shared by nitrosamine and volatile organics. Cadmium, also present in cigarette smoking, can act by inhibiting DNA repair mechanisms and by increasing cell-methylation and ROS production, thus facilitating carcinogenesis.
In what regards tobacco diffusion among general population, it was globally estimated that there were 1.14 billion active smokers in 2019 [6]. Although, over the last past decades, tobacco consumption changed: in Western countries, for instance, a consistent reduction in smokers thanks to the adoption of anti-tobacco public health measures was reported, even if an increased incidence has been described among women and the youth with a wide spread of new tobacco products. These latter conditions probably could be related to some observed changes in cancer histologies, for instance, with a lower incidence of squamous cell carcinoma and a higher percentage of adenocarcinoma for LC [22,23]. On the contrary, in low-income countries, tobacco smoking epidemy is still increasing, presenting a consistent public health issue [24].
Over the years, several studies confirmed the strong correlation between LC and the number of cigarettes smoked per day and the smoking years amount, highlighting an increased mortality of two–three times among middle-aged smokers (30 to 69 years old) compared to never smokers [23]. In 2004, Crispo et al., in a multicenter international case–control study, confirmed the role of cigarette smoking on LC onset, finding a cumulative risk of 14–16% for developing LC by age 75 for continuing smokers (at least 1 cigarette per day for at least 1 year) and of 20–25% for heavy smokers (at least 25 cigarettes per day) [25].
Due to the established tobacco-related morbidities, a program of tobacco cessation should be always considered, even after a cancer diagnosis, due to the several benefits of quitting smoking. This has been evaluated by the US Surgeon General’s Report for 2020, which established an improved survival and more in general significant benefits to non-cancer-related health outcomes after tobacco quitting, even for cancer patients. These data were, furthermore, confirmed by a recent meta-analysis of Caimi et al.: by analyzing 21 studies, they found an improved overall survival (OS) for patients who quitted smoking around a LC diagnosis with a summary relative risk (RR) of 0.77 (95% CI: 0.66–0.90) for NSCLC and 0.75 (95% CI: 0.57–0.99) for SCLC [26].
Even considering the strong evidence, in clinical practice, an appropriate smoking cessation program is not uniformly offered to patients at the time of a cancer diagnosis. This is the reason why the International Association for the Study of Lung Cancer (IASLC) has recently released a position statement in this regard. In particular, it recommends the evaluation of patient’s smoking status at the time of diagnosis and, whenever necessary, the integration of a smoking cessation program to all the other cancer cares and treatments [27].
Due to the carcinogens released by tobacco smoking, it is, nowadays, a well-established risk factor also for urothelial cancers (both bladder and upper tract urothelial cancers) and renal cell cancer, being responsible for a 2.5 times higher risk [28,29,30]. This higher risk is also influenced by the number of daily cigarettes and smoking duration without any differences between men and women [31,32]. The estimated latency after smoking initiation and bladder and upper tract urothelial cancers onset is around 20–30 years, and this can explain the latency rates of incidence compared to past smoking prevalence in the general population. Similarly to LC, after smoking cessation, the risk of bladder and upper tract urothelial cancers decreases by around 30%; in this regard, a relative benefit that patients already diagnosed with urothelial cancers may achieve in terms of cancer recurrence and time to progression in case of smoking cessation was also found [33,34]. The role of tobacco smoking in prostate cancer is much more elusive [35], even if recent meta-analyses and systematic reviews have reported a correlation between a worst prognosis for prostate cancer patients with a history of tobacco assumption, in terms of tumor volume, extra-capsular extension, and mortality [36]. Different mechanisms can explain this worse trend, including inflammation and CpG hypermethylation, both highly correlated to tobacco smoking and prostate cancer [37,38].
Second hand smoke, classified as class one carcinogen both in side stream and exhaled mainstream forms, has historically been associated with LC onset when it was observed as a major risk of LC diagnosis in smokers’ wives already in 1981 [39,40]. Similar results were achieved by a comprehensive meta-analysis conducted in 2007 and based on 55 studies; a 27% higher risk of LC was observed for women exposed to second hand smoke [17]. Evidence about the correlation of second hand smoke and urothelial cancers are much weaker, with a great heterogeneity between different studies, probably due to dose exposure differences, and thus more studies are required [41,42].
This entry is adapted from the peer-reviewed paper 10.3390/cancers15102836
This entry is offline, you can click here to edit this entry!
