1. Introduction
The small intestine has a huge surface area that is further enhanced by villi and microvilli to facilitate the digestion and absorption of nutrients. The expanded surface area of the small intestine increases the likelihood of exposure to pathogens in the lumen. The small intestine must balance the need for nutrient absorption with the ability to ward off pathogens [
58]. The majority of the immune cells in the body reside in the mucosa-associated tissues and the mesenchymal tissues of the gastrointestinal tract (GIT) [
59]. The gut-associated lymphoid tissues (GALT) play a vital role in the development of the immunity of the entire body, as most of the antigens that get into the body are transported to the GIT for processing by its innate immunity before being delivered to the adaptive immunity [
30,
59,
60].
A healthy life depends on maintaining the small intestine’s structural integrity and normal physiological function. Both the structural integrity and normal physiological function of the small intestine are dependent on the continuous generation of new IECs by the Lgr5
+ ISCs to replace senescent ones [
2,
61,
62,
63,
64]. A combination of the surface epithelial cells and junctional proteins provides a continuous physical barrier that is augmented by mucins [
5,
7]. The microbiota of the gut is also critical for the development and sustenance of the innate immunity of the small intestine [
65,
66,
67,
68,
69,
70]. Bacteria compete among themselves to maintain a healthy balance, while some of the viruses in the lumen of the small intestines are bacteriophages and take part in the control of potentially pathogenic bacteria [
7,
27,
71].
2. Intestinal Epithelial Cells and Innate Immunity
Almost all the IECs play a critical role in the innate immunity of the small intestine. Although all of them except the Paneth cells have a short lifespan of around 3–5 days, they are replaced quickly following their death. Regular replacement of the surface IECs ensures an intact physical barrier. Antimicrobial peptides and proteins that are secreted by the intestinal epithelial cells (IECs) are responsible for most of the chemical defence. Antimicrobial peptides are the most effective weapons against an overgrowth of pathogens and the subsequent bridge of the innate immunity of the small intestine [
14,
16,
72,
73].
2.1. The Role of Paneth Cells in the Innate Immunity of the Small Intestine
Paneth cells are among the derivatives of the Lgr5
+ ISCs in the small intestine and the small intestine’s major source of antimicrobial peptides and proteins [
62]. Paneth cells control the microbiota in the lumen of the small intestine and the proximal parts of the large bowel. The antimicrobial peptides from Paneth cells bathe and sterilise the area where the rapidly dividing and highly vulnerable Lgr5
+ ISCs are found [
2,
64,
73].
Matured Paneth cells are found at the base of the intestinal crypt of Lieberkühn in the entire small intestine from the duodenum to the terminal ileum [
74,
75]. Paneth cells start appearing in the small and large intestines of embryos at the end of 12 weeks, and their number increases significantly in the small intestine after 36 weeks [
73,
76]. The adult colon contains few, if any, Paneth cells. Goblet cells and other cells, instead of Paneth cells, are responsible for the antimicrobial activities in the colon [
76]. Metaplastic Paneth cells may appear in the colon and other sites in patients who have, for example, IBD [
77]. The number of Paneth cells increases during childhood and a full complement for an individual is reached later in life [
78]. Each intestinal crypt in an adult ultimately contains around 5–15 Paneth cells [
79]. The number of Paneth cells per intestinal crypt is, however, variable, as their density increases downwards. The highest density of Paneth cells in a healthy state is found in the ileum [
80].
The HD-5 is responsible for most of the antimicrobial activities in the small intestine [
13,
14,
74,
81]. The other antimicrobial peptides and proteins that are secreted by the Paneth cells include human α-defensin 6 (HD-6), lysozyme and phospholipase [
13,
82]. Although the other IECs such as the absorptive enterocytes, goblet cells and tuft cells survey and regulate the gut microbiota, Paneth cells are the most critical for innate immunity in the small intestine, as they secrete the largest amount of the most potent antimicrobial peptides and proteins [
16,
78]. In addition, the localisation of matured Paneth cells at the base of the intestinal crypts ensures the maximal concentration of antimicrobial peptides for the defence of the highly active but most vulnerable Lgr5 ISCs [
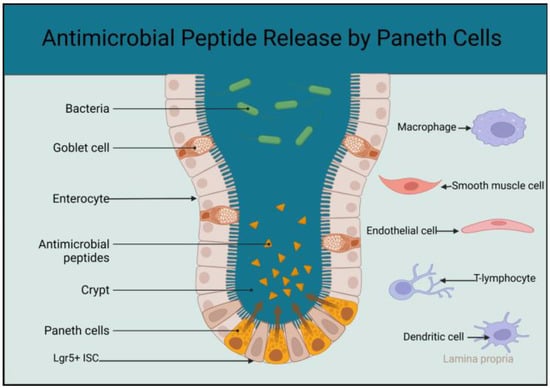
78] (
Figure 1).
Figure 1. Schematic diagram showing the localisation of matured Paneth cells at the base of the intestinal crypt and higher concentration of antimicrobial peptides at the stem cell zone. The sketch also includes cells in the lamina propria that interact with the Paneth cells to support and regulate activities of the Lgr5+ ISCs (created using BioRender.com accessed on the 25 April 2023).
Paneth cells are pyramidal in shape. They have a broader base where their nucleus is situated [
82]. Paneth cells are among the secretory derivatives of the Lgr5
+ ISCs, and their cytoplasm contains several organelles, which include the endoplasmic reticulum and Golgi apparatus. The apical area of Paneth cells has eosinophilic granules that contain, among other constituents, HD-5, HD-6, lysosome, growth factors, Wnt signals and cytokines [
74,
82]. Paneth cells release their constituents after the detection of pathogens through their pathogen recognition receptor system. Paneth cells continually sample the microbiota in the lumen of the small intestine to prevent dysbiosis [
82,
83,
84]. Paneth cells also secrete antimicrobial peptides and other products following a stimulus from the brain via the cholinergic system. The mere sight or smell of food may also lead to the activation of the Paneth cells. Additionally, Paneth cells sample the composition of nutrients in the food following ingestion [
7] (
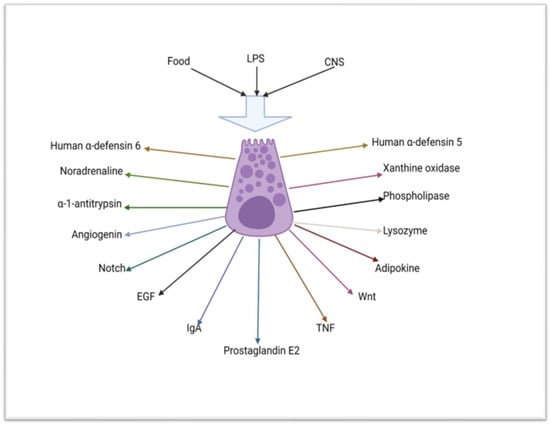
Figure 2).
Figure 2. Schematic diagram depicting the mechanism of stimulation and some of the substances that are secreted by the Paneth cell (created using BioRender.com 9 of April 2023). LPS = lipopolysaccharide, CNS = central nervous system, Notch = neurogenic locus notch homolog protein 2, EGF = epidermal growth factor, IgA = IgA immunoglobulin, TNF = tumour necrosis factor, Wnt = wingless/integrated.
Some of the antimicrobial proteins and peptides secreted by the Paneth cells are stored as zymogens in the cytoplasmic granule and are activated just before release [
82]. Once activated, HD-5 can kill all bacteria, fungi and parasites and some viruses [
85]. Paradoxically, HD-5 can enhance proliferation of certain viruses [
12,
82]. Human defensin 5 is lipophilic and kills pathogenic bacteria by creating pores on the cell membrane, which increases the permeability, thus making the bacteria swell up and subsequently burst [
12,
17,
19]. Human α-defensin 6 does not have anti-microbiocidal activity but is able to create nanonets around pathogens, thus trapping and containing them [
84] (
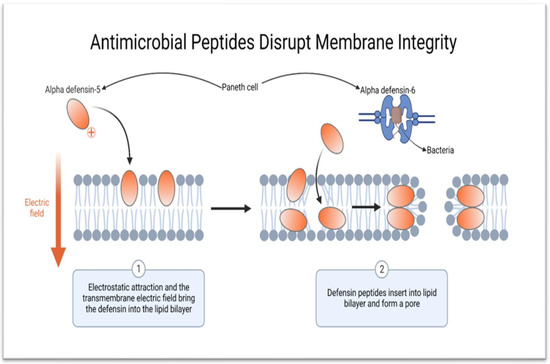
Figure 3).
Figure 3. Schematic diagram showing the mechanism of action of HD-5 and HD-6. HD-5 kills bacteria and other microorganisms by creating pores on the cell membrane, while HD-6 forms nanonets to entrap pathogens (created using BioRender.com on 9 of February 2023).
Lysozyme and IgA, also secreted by the Paneth cells, add to the antimicrobial activities in the lumen of the small intestine [
80]. The other functions of the Paneth cells that are critical for the maintenance of robust innate immunity in the small intestine include the regulation of proliferation and differentiation of the Lgr5
+ ISCs, metabolic support of the Lgr5
+ ISCs and liaison with cells involved in adaptive immunity [
2,
12,
59,
60,
62,
83,
85,
86]. Abnormality in the number or function of Paneth cells is seen in numerous diseases, including viral infections [
80], necrotising enterocolitis [
76], prolonged use of total parental nutrition [
87], starvation [
88], Crohn’s disease [
15,
77,
83,
89,
90,
91], smoking [
90] and ageing [
8]. A change in the total number of Paneth cells or a deterioration in the quality of their function may also occur during chronic HIV infections [
92].
2.2. Absorptive Enterocytes, Goblet Cells, Tuft Cells, M-Cells and Junctional and Innate Immunity of the Small Intestine
Goblet cells augment the antimicrobial defence by secreting mucous made up of mucins, water and trefoil factors. MUC2 is the major constituent of the mucus and helps to form a carpet of mucus that maintains a higher concentration of antimicrobial peptides in the area adjacent to the surface of the IECs [
74]. Secretory mucus is an essential nutrient for some commensal organisms. The absorptive enterocytes are the most abundant IECs [
2].
Absorptive enterocytes have pathogen recognition receptors, which they use to sample the contents in the lumen of the small intestine and therefore participate in innate gut defence [
9]. Like any other epithelial cell throughout the body, the absorptive enterocytes secrete β-defensin and not HD-5 or HD-6. Other IECs that participate in the regulation of the microbiota in the lumen of the small intestine are the tuft cells and the M-cells [
58,
93,
94,
95,
96]. The tuft cells sample the gut microbiota like the Paneth cells but to a limited extent [
96,
97,
98]. Secretions from the tuft cells are limited to cytokines [
99]. Tuft cells have not been shown to secrete antimicrobial peptides, growth factors or catecholamines [
99]. The junctional proteins also add to the innate immunity of the small intestine [
5,
7,
27].
3. Gut Microbiota and Innate Immunity in the Small Intestine
The gut lumen contains numerous species of bacteria, viruses, fungi, parasites and archaea [
100]. Around 10
14 bacteria reside in the colon, but the small intestine contains fewer microorganisms [
78]. Colonisation of the gut starts in utero and increases during childbirth [
101,
102]. Further changes in the gut microbiota composition occur during breastfeeding and weaning [
101,
102]. The normal gut flora of an individual is established during early childhood or adolescence. Once established, the gut microbiota is involved in the regulation of the physiological function and innate immunity of the small intestine [
101,
103]. The gut microbiota also influences the proliferation and differentiation of the Lgr5
+ ISCs and assists with the digestion of food [
28]. Some of the commensals in the microbiota help process nutrients such as vitamins and short-chain fatty acids (SCFAs) like acetate and butyrate [
3,
28]. Complex dietary fibres would be difficult to digest and absorb without the assistance of commensal bacteria [
3,
28].
Certain species of bacteria help sustain the prevailing anti-inflammatory state to prevent damage to the intestinal epithelium, increased permeability, translocation of endotoxins and chronic systemic inflammation [
32]. The other roles of the commensal organisms include preventing excessive production of ROS or RNS [
30]. Furthermore, some of the commensal bacteria in the microbiota possess quorum sensors and secrete bacteriocins to kill the pathogenic species [
70]. In addition, several species of bacteria can influence the cells involved in adaptive immunity in the lamina propria of the small intestine [
30]. Several bacteria can modulate the hormonal milieu in the GIT and the entire body by participating in the microbiota-gut-brain axis. In return, commensal organisms depend on the nutrients in the diet, residue following digestion of nutrients and mucins secreted by goblet cells [
104].
More than 80% of the bacteria in a healthy adult human belong to the Firmicutes and Bifidobacterium phyla [
105]. Changing diet, use of antibiotics, chronic illness and excisional or bypass surgery in the GIT may lead to dysbiosis [
106,
107]. Dysbiosis may involve the entire length or certain niches along the GIT. Dysbiosis commonly leads to a reduction in the diversity of the microbial species and the dominance of pathogenic organisms like the Bacteroides [
3,
27,
30,
32,
78]. Dysbiosis not only involves bacteria but may also include the virome [
100]. Dysbiosis and the emergence of pathogenic species may lead to an increase in the production of ROS [
108]. High levels of ROS in the lumen of the gut can damage the intestinal epithelium [
108]. Concomitantly, pathogenic gram-negative or gram-positive bacteria may initiate an inflammatory response by releasing pro-inflammatory lipopolysaccharides or peptidoglycan, respectively [
32,
109] (
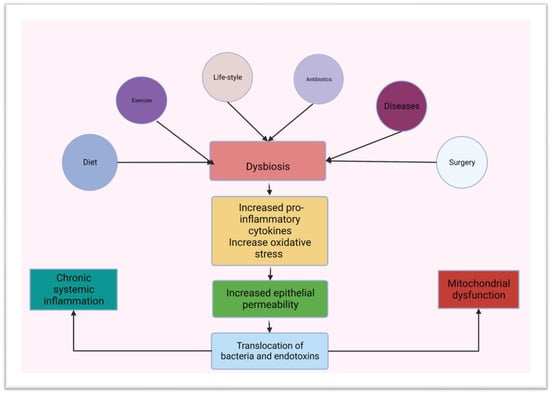
Figure 4).
Figure 4. Schematic diagram showing predisposing factors for dysbiosis and a link between dysbiosis, chronic inflammation and mitochondrial dysfunction (created using BioRender.com on the 3 of March 2023).
Inflammation of the epithelium of the small intestine increases its permeability, leading to the translocation of bacteria and their products [
29]. Ongoing translocation of bacteria and endotoxins causes endotoxaemia and chronic systemic inflammation [
4]. Chronic systemic inflammation creates an environment that is obesogenic and diabetogenic [
106]. Chronic systemic inflammation also increases the generation of ROSRNS from mitochondria in tissues and organs throughout the body [
34,
78]. Some cells in organs throughout the body such as the β-cells of the islets of Langerhans in the pancreas have a limited capacity to neutralise reactive species, which can perpetuate the damage to their mitochondria [
30].
The high level of glucose in the blood and tissues creates a mismatch between the aerobic glycolysis and the oxygen-dependent tricarboxylic acid cycle, which increases the production of ROS and RNS [
42,
45,
109]. Chronic inflammation also impairs mitophagy in the mitochondria and affects the ability of the mitochondria to recycle and preserve essential constituents through either fusion or fission [
48]. Dysbiosis, increased ROS production, increased gut permeability, translocation of bacteria and chronic systemic inflammation are the pathological basis of common non-communicable diseases like obesity [
110], depression [
111], cancer [
110,
112,
113] and DM [
108,
114,
115]. Over 90% of individuals who have DM are obese [
101]. The adipokines from the adipose tissue in individuals who are obese sustain and worsen the inflammatory process [
39,
113].
This entry is adapted from the peer-reviewed paper 10.3390/app13116605