Liquid crystals are generally referred to as substances that blend the structure and properties of solid and liquid states; they share with liquids the ability to flow but also exhibit some structural arrangement similarities with solids. With so many compounds synthesized, liquid crystals containing metals, also known as metallomesogens, have become a major subject of study. The incorporation of metal into organic matrices enhances and induces unique magnetic, spectroscopic, and redox properties of the resulting materials. At least one liquid crystalline complex has been reported in the literature for most metals.
1. Introduction
Copper(I) complexes have not been studied to a great extent as luminescent materials in the liquid crystalline state, but they do possess luminescence properties with great potentials [13,14,15,16,17,18,19,20,21,22]. Copper, which is somewhat abundant and affordable, is a suitable alternative to noble metal complexes [23,24]. The ratio of triplet to singlet excitons is 3:1; consequently, for luminescent materials to be used in OLEDs, they should essentially be able to harvest all the excitons. Because copper(I) complexes exhibit various metal-to-ligand charge-transfer (MLCT) behaviors, they can induce spin orbital coupling of the triplet and singlet states, leading to small energy separations between the energy levels [25]. This allows for reverse intersystem crossing (RISC), i.e., singlet harvesting, resulting in thermally activated delayed fluorescence (TADF) [26]. Therefore, liquid crystals based on copper(I) complexes have considerable promise for producing effective luminescent materials for a wide range of optical or electro-optical applications due to the large diversity of possible structures, including mononuclear or polynuclear complexes, and the great potential of emission behavior. In addition, the range of coordination geometries (such as linear, plan-trigonal, or tetrahedral) combined with the ligands’ structural design provide a significant benefit for controlling the LC properties, including their enhanced thermal stability and mesophase type related to both calamitic and discotic materials.
2. Copper(I) Metallomesogens with Sulfur-Containing Ligands
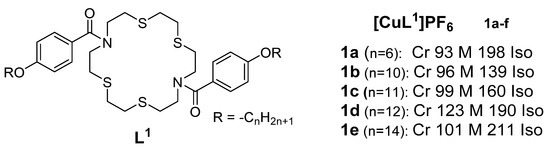
A series of cationic macrocyclic copper(I) complexes based on non-mesogenic bis[4-(n-alkyloxy) benzamide derivatives of 1,10-diaza-4,7,13,16-tetrathiacyclooctadecane was reported in 1994 by Ghedini et al. [
32]. The studied complexes had transition temperatures from the solid to liquid crystalline states ranging from 93 to 123 °C. Complex
1e (
Figure 1) demonstrated the clearest mesomorphism among the series, with sufficient thermal stability, so the research group focused their X-ray analysis on it, and the study revealed an X-ray diffraction pattern consistent with a disordered layered structure associated with a smectic phase of A or C type.
Figure 1. Copper(I) complexes from azamacrocycle derivatives. Transition temperatures are in °C [
32].
Alkyl thiolates (R-SH) are strong ligands that can bind to metals via the donor S atom to obtain metal thiolates. In 1999, Espinet et al. [
33] reported the preparation of a series of copper(I) thiolates, [CuSC
nH
2n+1], where n = 4, 6, 8, 10, 12, 14, 16, and 18. X-ray diffraction analysis of the polycrystalline sample revealed a layered structure in the solid state, contrary to previously reported work by Dance et al. (1991) [
34]. However, the authors suggested that the discrepancies might have been due to the methodology used, indicating that the preparative method of the complex influences its stability towards oxidation. The textures of the complexes show a columnar mesophase based on stacking of cyclic [Cu
4(µ
2-SC
nH
2n+1)
4] aggregates, with transition temperatures ranging from 56 to 210 °C.
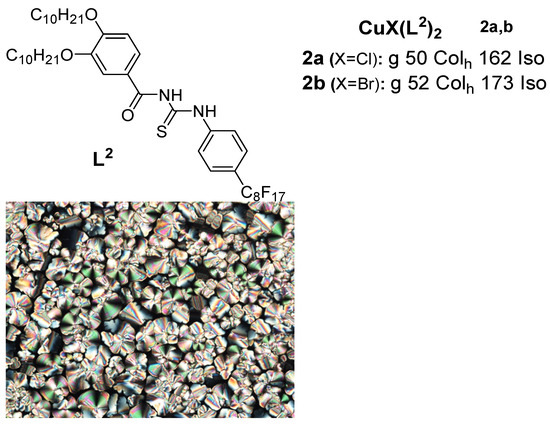
A new class of copper(I) metallomesogens based on copper(I) halide complexes with thiourea-based ligands with long-chain alkoxy groups and a perfluorooctyl group were reported in 2018 by Ilis and Cîrcu [
35] (
Figure 2). They found no liquid crystalline behavior for the ligand but observed a hexagonal columnar phase for both complexes
2a and
2b at high temperatures above 100 °C via a combined study of POM, DSC, and XRD, while the thermal stability studied by TGA indicated a higher stability (180 °C) for the corresponding copper(I) complexes compared to that of the BTU (160 °C) ligand.
Figure 2. Copper(I) complexes with benzoylthiourea ligands. Transition temperatures are in °C. Inset: POM image of the Col
h phase of
2a at 85 °C [
35].
3. Copper(I) Metallomesogens with N-Donor Ligands
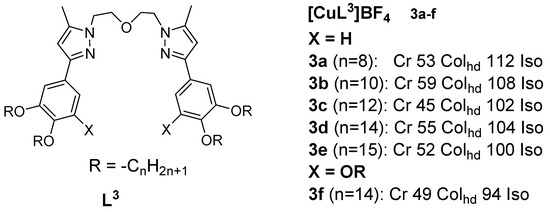
Many copper(I) complexes with N-donor ligands integrating pyrazole, 2,2’-bypiridine, 1,10-phenantroline moieties, or Schiff bases have been described to date. Copper(I) metallomesogens with three-coordinate geometry were first reported by Lin et al. in 2001 [
36]. The complexes were derived from bis{2-[3’-(3’’,4’’-dialkoxyphenyl)-5’-methyl-1’-pyrazolyl]ethyl} ethers and from bis{2-[3’-(3’’,4’,5’-trialkoxyphenyl)-5’-methyl-1’-pyrazolyl]ethyl} ethers. These novel complexes were obtained by complexing the ethers with [Cu(MeCN)
4]BF
4 (
Figure 3). The reported copper(I) complexes with four or six alkoxy chains exhibited liquid crystalline behavior and are characteristic of columnar discotics. DSC analysis results indicated a higher enthalpy for the melting transitions at lower temperatures and a relatively lower enthalpy for the isotropization transitions at higher temperatures. The attachment of an additional alkoxy chain on the terminal benzene ring resulted in lower transition temperatures, while preserving the mesophase type (Col
hd).
Figure 3. Copper(I) complexes with ether-type ligands. All temperatures are in °C [
36].
Schiff bases are a diverse group of compounds formed by the nucleophilic substitution reaction of an aldehyde or ketone with an amine. These compounds are characterized by the presence of a double bond linking carbon and nitrogen atoms, the functionalities of which are generated in many ways to combine a variety of alkyl or aryl substituents useful for the design of liquid crystalline materials, either organic or metallomesogens [
37,
38,
39,
40,
41,
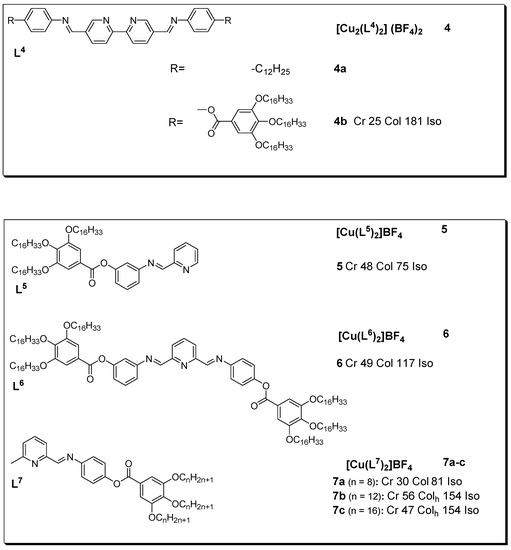
42]. The lone pair of electrons on nitrogen provides a basis for making complexes with metals, including copper(I). Diverse liquid crystalline copper(I) complexes have been prepared with these ligands; the resulting complexes are indicated in
Figure 4. Dinuclear copper(I) complexes (
4a and
4b) with Schiff bases based on the α,α’-imino-substituted 2,2’-bipyridine unit were developed by El-ghayoury et al. [
43]. The two ligands adopt a rather unusual coordination mode in which the central unit bridges the two copper(I) centers. The tetrahedral surrounding is completed by the coordination of the two imino groups of each Schiff base ligand with the iminopyridine fragment chelated in a cis fashion to provide a symmetric structure. Complex
4a is non-mesomorphic, owing to the unsuitable balance between the aliphatic chains and the aromatic core. The optical textures observed upon slow cooling of the isotropic melt of complex
4b clearly indicate the existence of a viscous columnar phase at temperatures as low as 25 °C.
Figure 4. Copper(I) metallomesogens with Schiff bases. All transition temperatures are in °C (transition temperature for
4a not reported) [
43,
44,
45].
Although non-mesogenic, upon complexation with copper(I), free ligands L
5 and L
6 showed mesogenic character, as described by the DSC and POM experiments. The results indicated that complex
6 showed high stability after several heating cycles relative to complex
5, which was thought to be due to the lack of substituents at position 6. In addition, the optical textures observed for complex
6 during slow cooling from the isotropic melt are typical of a columnar phase (with pseudo-focal-conic textures [
45]. Similarly, Douce and coworkers prepared new complexes (
7a–
c) by modifying the organic ligands to enable scrutiny of the nature of the packing in the liquid crystalline phase. In their work, the authors reported the preparation of new ligands with various chain lengths (n = 8, 12, and 16) bearing an additional methyl fragment in the α-position of the pyridine ring in order to protect the copper complex (
5) against oxidation and decomplexation. The prepared complexes displayed hexagonal columnar mesophases with a transition temperature range of 30 to 56 °C [
44].
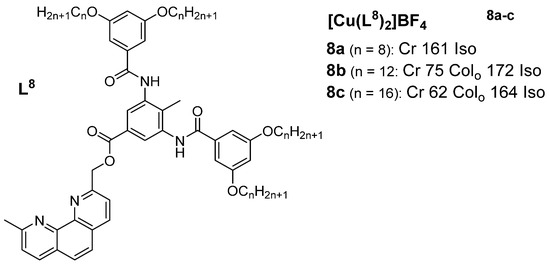
A study by Ziessel et al. (2004) [
46] presented mesomorphic materials based on copper(I) complexes with phenanthroline-based ligands (
Figure 5). The authors aimed to engineer a structural framework with additional supramolecular binding factors (hydrogen bonding) so as to stabilize the mesophase both as a free ligand and within the complex. The thermotropic properties of the ligands and complexes of the phenanthroline derivatives were investigated via a combination of POM, DSC, and XRD methods. The ligand used for complexes
8b and
8c showed distinct cubic and disordered lamellar mesophases at different temperature regions in the reported XRD measurements. Neither the ligand with the shortest chain (n = 8) nor the corresponding copper(I) complex (
8a) displayed any mesomorphic behavior in the investigated temperature region, but as expected, the related copper(I) complexes with longer chains (
8b (n = 12) and
8c (n = 16)) showed mesomorphic behaviors, displaying mesophases characteristic of an oblique columnar phase. In effect, the complexation of the metal center with the organic ligand resulted in a distinct change in the mesomorphic properties. It was observed that the mesophase stability was enhanced upon coordination with copper(I), as reflected by the large increase in the clearing temperature by nearly 50 °C, whereas the melting temperature remained almost the same for the ligands, as well as for the corresponding complexes.
Figure 5. Copper(I) complexes with phenanthroline-based ligands. Temperatures are in (°C) [
46].
This entry is adapted from the peer-reviewed paper 10.3390/chemistry5010046