Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Applied
Coumarins are secondary plant metabolites widely distributed in higher plants, bacteria, fungi, and sponges. This great structural diversity of these natural compounds and their synthesized derivatives enables their wide range of pharmacological activities, such as antioxidant; antibacterial; antifungal; anti-human immunodeficiency infection; anti-tubercular; and anti-cancer activities. There are also many reports about their effectiveness against plant pathogenic pests (phytopathogenic fungi, bacteria, nematodes, and insects).
- coumarins
- pesticides
1. Introduction
The control of fungal pathogens and pests has vital importance for the protection of crops and food provision worldwide. Organic compounds are still the major active components of plant protection products. Although pesticides in agronomy exert powerful effects on disease control management, the long-term their unreasonable use led to serious environmental problems inducing successive pesticide-resistant pathogen development, disrupting soil ecological balance, and causing environmental and human health. There are among the most common pollutants in one-fifth of the Earth’s land. Plant protection products pollute terrestrial and aquatic ecosystems, and every year millions of people are exposed to pesticides [1]. Daily exposure to pesticides has numerous consequences for human health. Pesticide exposure has numerous health consequences. Pesticides could induce tumors in the liver, lungs, stomach, kidneys, skin, and stomach [2,3]. Also, exposure to pesticides has been associated with dermatological, gastrointestinal, neurological, respiratory, reproductive, and endocrine symptoms of diseases [4]. Drug resistance and environmental and health hazards indicate the urgent need for novel active compounds. These compounds must be highly specific with a broad-spectrum mode of action, as well as environmentally and toxicologically acceptable [5]. In order to limit pesticide harmful effects, the European Parliament and the Council issued Directive 2009/128/EC that promotes integrated pest management with priority for plant protection products. This Directive has the fewest side effects on human health, non-target organisms, and the environment [6]. Coumarins are secondary plant metabolites widely distributed in higher plants, bacteria, fungi, and sponges [7]. Depending on the substitution of the 1-benzopyran-2-one skeleton, coumarins can be divided into several types: simple coumarins, furanocoumarins, pyranocoumarins, and other coumarins (Figure 1). This great structural diversity of these natural compounds and their synthesized derivatives enables their wide range of pharmacological activities, such as antioxidant [8,9,10]; antibacterial [11,12,13,14,15,16,17]; antifungal [18,19,20]; anti-human immunodeficiency virus (HIV) infection [21,22]; anti-tubercular [23]; cytotoxicity [24]; and anti-cancer activities [25,26,27,28,29].
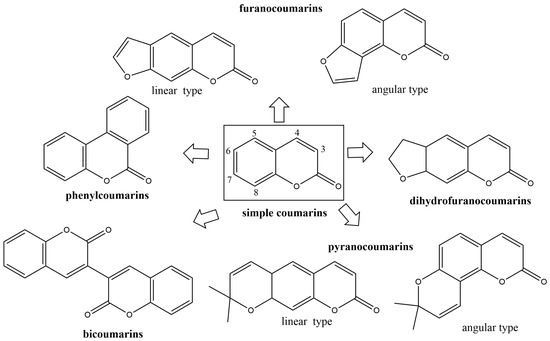
Figure 1. The main groups of natural coumarins.
There are also many reports about their effectiveness against plant pathogenic pests. These secondary metabolites are natural protection agents against environmental enemies and competing plants, therefore they are called allelochemicals. Allelochemicals are biocommunicators that act in a natural mixture of active components, while single compounds are not active [7,30,31]. Coumarin derivatives have been reported as strong agents against phytopathogen fungi, such as: Botrytis cinerea [32]; Moniliophthora perniciosa [33]; Colletotrichum gloeosporioides, Fusarium oxysporum, Valsa mali [34]; Macrophomina phaseolina and Sclerotinia sclerotiorum [35,36]. Coumarins have also antimicrobial potential against phytopathogens: Ralstonia solanacearum [37]; Agrobacterium tumefaciens [38]; Pseudomonas aeruginosa [39]. Nematicidal activity has been demonstrated for several simple coumarins, furanocoumarines, and dicoumarols, and their skeletons have been used for the development of new efficient nematicides against plant parasitic nematodes: Meloidogyne incognita, Ditylenchus destructor, Bursaphelenchus xylophilus, Bursaphelenchus mucronatus, and Aphelenchoides besseyi [40,41].
2. Naturally Occurring Coumarins and Their Role in Plants
2.1. Biosynthesis and Distribution of Coumarins in Nature
As a family of benzopyrones (1,2-benzopyrones or 2H-1-benzopyran-2-ones), coumarins are widely distributed throughout nature. The benzopyrone framework is an electron-rich system with favorable charge-transport properties. Therefore, they are characterized by UV light absorption, resulting in a characteristic blue fluorescence. Besides their role in iron mobilization and uptake by plant roots, natural coumarins have a role in environmental stress responses. Also, they participate in the defense against plant pathogens, acting as phytoanticipins, or phytoalexins, which are produced upon infection and are typically not present in healthy tissues. Their increased accumulation on plant tissue is a response to the application of a molecule that triggers the hypersensitivity response in the plant (elicitor) or plant hormones [60].
Since coumarins act as signaling molecules that regulate the interaction between commensals, pathogens, and plants, they could be used as biopesticides. Endophytes such as bacteria or fungi have the ability to produce some of the secondary metabolites. Thus, coumarin isofraxidin was synthesized by the fungal endophyte Biscognia uxiacylindrospora, and identified in host plants Siberian ginseng (Acanthopanax senticosus) and Apium graveolens [61]. Infection by pathogenic bacteria can induce the synthesis of coumarin compounds around plant roots and stems to immunize the plant against pathogen invasion and propagation. Inoculation of Arabidopsis thaliana by the plant pathogen Dickeya spp. strains induced coumarin accumulation and plant resistance to pathogens [62].
Treatment of ryegrass (Lolium multiflorum Lam.) with coumarins stimulates the colonization of beneficial flora in the root rhizospheric microbial community. Rhizosphere microorganisms enhance plant nutrient absorption, coordinate growth, and improve environmental adaptability [63]. The secretion of coumarins from Arabidopsis thaliana roots under soil iron deprivation stimulates the bacterial root microbiota to improve plant adaptation to iron-limiting soils [64]. A strain of Aspergillus synthesizes 4-hydroxycoumarin and dicoumarol [65,66].
Naturally occurring coumarins are mostly distributed in plants seeds, flowers, leaves, roots, and stems in more than 40 different families including Apiaceae, Rutaceae, Asteraceae, Fabaceae, Oleaceae, Moraceae, and Thymelaeaceae [67]. The natural coumarins are derivatives of 2H-1-benzopyran-2-one, and they are classified into six groups: simple coumarins; furanocoumarins (linear and angular type); dihidrofuranocoumarins; pyranocoumarins (linear and angular type); phenylcoumarins; and bicoumarins (Figure 1) [68]. Angiospermaes are rich in simple coumarins, followed by furanocoumarins and pyranocoumarins. The most diverse sources of coumarins are plants families Apiaceae and Rutaceae containing five different types of coumarin derivatives (simple coumarins, lineal furocoumarins, angular furocoumarins, lineal pyranocoumarins, and angular pyranocoumarins) [30].
Simple coumarins are derived by biosynthesis from shikimic acid, via cinnamic acid. They are the most common in all angiosperms, especially in Oleaceae and Asteraceae. A key step in the biosynthesis of simple coumarins is ortho-hydroxylation of cinnamates that branch off from lignin biosynthesis. The gene required for the production of feruloyl coenzyme A (CoA) is CCoAOMT1. It also participates in the biosynthesis of lignin and simple coumarin scopoletin in Arabidopsis roots. A key enzyme involved in the biosynthesis of simple coumarins is 2-oxoglutarate-dependent dioxygenase (2OGD), which is encoded by 2OGD genes. Thus, the gene AtF6_H1 encodes otho-hydroxylase activity to feruloyl coenzyme A, and its deficient mutation causes a significant reduction in scopolin accumulation. 2OGD gene RgC2′H formation of furanocoumarins in Ruta graveolenes [69]. Beta-glucosidase (BGLU) genes are regulatory genes responsible for coumarin biosynthesis in Melilotus species and differences in their expression result in coumarin content diversity among Melilotus species [70]. Coumarin biosynthesis genes are also activated after foliar pathogen infection to create a microbial soil-borne legacy that primes plants for defenses. Coumarin biosynthesis genes, such as root-specific transcription factors myb72 and f6′h1 are also activated in the plant Arabidopsis thaliana after foliar pathogen infection with downy mildew pathogen Hyaloperonospora arabidopsis (Hpa), for the creation of a microbial soil-borne legacy (SBL) that primes plants for defenses [71]. Since scopoletin selectively inhibits the soil-borne fungal pathogens Fusarium oxysporum and Verticillium dahliae, the study of Stringlis et al. [72] has shown that during infection, probiotic root-associated microbes stimulate MYB72-dependent excretion of scopoletin. Armillarisin A (3-acetyl-5-hydroxymethyl-7-hydroxycoumarin) is a coumarin derivative extracted from the fungus Armillariella tabescens (Scop. ex Fr.) Sing [73]. Chlorinated coumarins, 6-chloro-4-phenyl-2H-chromen-2-one and ethyl 6-chloro-2-oxo-4-phenyl-2H-chromen-3-carboxylate, were identified in the polypore mushroom Fomitopsis officinalis [74].
2.2. Classification of Naturally Occurring Coumarins and Their Role in Plant Protection
2.2.1. Simple Coumarins
Simple coumarins act in plants’ interaction with biotic and abiotic environmental stress factors. Secretion of iron-mobilizing coumarins by plant roots is a crucial factor for improving iron bioavailability in crops, enabling them to grow in iron-depleted soils. In Arabidopsis, three coumarins with iron-mobilizing properties are found: fraxetin (7,8-dihydroxy-6-methoxycoumarin), sideretin (5,7,8-trihydroxy-6-methoxycoumarin), and esculetin (6,7-dihydroxycoumarin) (Figure 2 Their catechol moiety (two neighboring hydroxyl groups) is thought to be a crucial structural feature for iron mobilization in soil [75]. Scopoletin (7-hydroxy-6-methoxy coumarin) (Figure 2) is a simple coumarin that occurs in Arabidopsis thaliana [76], and many other plants [77]. Physiologically, scopoletin protects against stress. Thus, it was proved that scopoletin accumulates in Arabidopsis leaves after the attack of the fungus Phakopsora pachyrhizi, which causes Asian soybean rust disease [78].
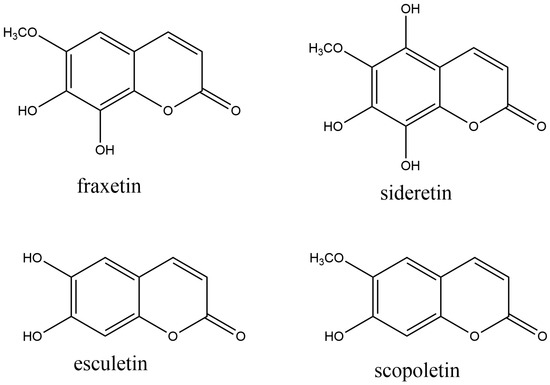
Figure 2. The most common naturally occurring simple coumarins.
In tobacco plants, scopoletin and its β-glucoside, scopolin, have physiological roles against stress, for example during tobacco mosaic virus infiltration [79]. Surangib B from Mammea longifolia inhibits mycelial growth of Rhizoctonia solani and Botrytis cinerea [80]. Umbelliferone (7-hydroxycoumarin) is a phytoalexin widely distributed within the Rutaceae and Apiaceae (Umbelliferae) families. It is an important phytoalexin that protects plants from pathogenic fungi, such as Fusarium culmorum [81], and the aerobic, Gram-negative, plant-pathogenic bacterium Ralstonia solanacearum [82].
Yang et al. [83] studied the antimicrobial activity of 18 natural compounds against Ralstonia solanacearum, the bacterium responsible for tobacco, tomatoes, and potatoes wilting in subtropical regions. The research showed that four coumarins, daphnetin, esculetin, umbelliferone, and xanthotol demonstrated stronger antibacterial effects than the standard treatment with thiadiazole and copper. A more detailed analysis showed that the enhanced antibacterial activity is due to the substitution at positions C-6, C-7, and C-8 of the coumarin nucleus. For this reason, they tested the activity of the hydroxycoumarins umbelliferone, esculetin, and daphnetin in the concentration range from 10 to 100 mg/L. Daphnetin (OH groups at positions C-7 and C-8) proved to be the most effective, esculetin (OH groups at positions C-6 and C-7) was somewhat weaker, while umbelliferone (OH group at positions C-7) showed the weakest activity. Thus, treatment of tobacco roots with umbelliferone prior to infection with R. solanacearum significantly reduced R. solanacearum biofilm formation, increasing resistance to disease [84]. Application of an elicitor of coumarin biosynthesis, salicylic acid to the roots of chamomile (Matricaria chamomilla) resulted in the accumulation of ambelliferone and herniarin (7-methoxy coumarin) in the leaves [85]. Herniarin suppressed R. solanacearum bacterial growth by destroying the bacterial cell membrane [86]. Also, plant-derived 6-methylcoumarin showed inhibitory effects against R. solanacearum, and suppressed tobacco bacterial wilt [87]. Derivatives of 3,4-dihydroisocoumarin isolated from endophytic fungus Lophiostoma sp., displayed antibacterial activities against Bacillus subtilis, Agrobacterium tumefaciens, R. solanacearum, and Xanthomonas vesicatoria [88].
Increased biosynthesis of coumarins, ayapin and scopoletin, has been observed in sunflowers (Helianthus annuus L.) during the attack of the sunflower beetle, Zygogramma exclamationis, which resulted in distracting further feeding of the beetle [89]. The coumarin (2H-1-benzopyran-2-one) proved effective against the green peach aphid Myzus persicae and friendly to the natural enemy of aphids, Harmonia axyridis and soil invertebrates, Eisenia fetida. The study implied that coumarin can be recommended as a selective and effective botanical aphicide friendly to non-target organisms. However, the environmental safety of a given insecticide must be estimated with further tests to clarify the mechanism of its action and efficacy [90]. Recently, five simple coumarin-based scaffolds (limetin-derivatives) were identified in Citrullus lanatus seeds, which possess significant bactericidal and fungicidal potential [91].
2.2.2. Furanocoumarins
Furanocoumarins are composed of a furan ring fused to a coumarin core (Figure 3).
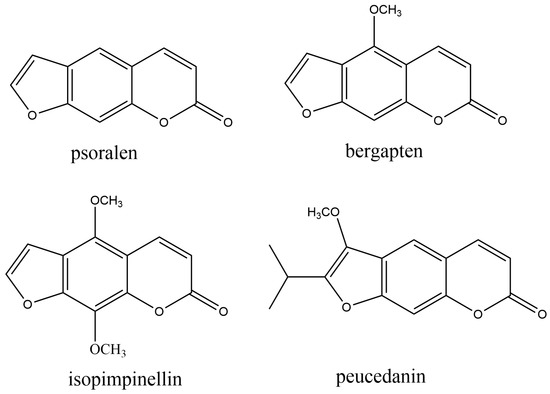
Figure 3. The most common naturally occurring furanocoumarins.
They are mostly present in plant families, including Apiaceae, Fabaceae, Moraceae, and Rutaceae. Their increased level in plants is a response to stress conditions, such as exposure to pathogenic fungi or to physical damage caused by occasional lesions or insect bites. They are involved in plant defense, acting against microorganisms, nematodes, phytophagous insects, herbivores, and plant competitors [92]. Linear furanocoumarins, such as psoralen, bergapten, isopimpinellin, and xanthotoxin, together with the angular dihydrofuranocoumarin athamantin, are antifeedants. Peucedanin (Figure 3) inhibits the growth of neonate larvae of Spodoptera litlis (Boisduval) (Lepidoptera: Noctuidae) [93]. Plants are a prominent source of novel nematicidal chemicals. Thus, furanocoumarins 8-geranyloxy psolaren, imperatorin, and heraclenin from the root extract of Heracleum candicans Wall., exhibited nematicidal effects against Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle and Pangrellus redivivus (Linn.) [94]. Also, bergapten and psoralen extract from Ficus carica L. leaves showed strong nematicidal activity against pine wood nematode (PWN), Bursaphelenchus xylophilus. Their nematicidal mechanism is probably based on the inhibition of amylase, cellulase, and acetylcholinesterase [95]. Ethanol extract from Chinese medicinal herb Notopterygium incisum rhizomes possessed strong nematicidal activity against two species of nematodes, Bursaphelenchus xylophilus and Meloidogyne incognita. The extract contained two furanocoumarins, colombienetin and isoimperatorin [96]. Essential oil and methanol extracts of parsley (Petroselinum crispum) [97], as well as ethanol extracts from Angelica pubescens Maxim. f. biserrata Shan et Yuan roots [98], exhibited promising nematicidal activity as a source of nematotoxic furanocoumarins.
A number of furanocoumarin compounds isolated from Semenovia transiliensis shoots have herbicidal activities [31]. Citrus plants produce simple coumarins and furanocoumarins to cope with herbivorous insects and pathogens [99]. Ramirez-Pelayo et al. [100] studied coumarin derivatives found in citrus peels. They isolated six coumarins (5-geranyloxy-7-methoxycoumarin, bergamottin, bergapten, isopimpineline, citropten, and oxypeucedanin hydrate) and tested their antifungal activity against Colletotrichum sp., a fungus causing fruit anthracnose. Their activity was compared with the simpler coumarins, umbelliferone, scoparone, and scopoletin. The test results showed that all six coumarins inhibited the growth of Colletotrichum sp. mycelia, and among them, bergapten and citropten proved to be the most effective. The research concluded that there is a synergistic effect between the individual coumarin components in the citrus peel extract.
2.2.3. Dihydrofuranocoumarins
The presence of dihydrofuranocoumarins in all plant parts, and especially the roots, is responsible for plants’ poisonous properties, such as Opopanax chironium (Apiaceae) [101] and Opopanax hispidus (Friv.) Griseb. [102]. It was found that dihydrofuranocoumarin xanthoarnol from the plant Xanthoxylum arnottianum (Rutaceae) showed an inhibitory effect on the germination of conidia of the parasitic fungus [103].
2.2.4. Phenylcoumarins
4-Phenylcoumarins protect plants against pests and fungi. Thus, 5,7-dimethoxy-4-p-methoxyphenylcoumarin and 5,7-dimethoxy-4-phenylcoumarin (Figure 4) were found in the microorganism Streptomyces aureofaciens, which was isolated from ginger root tissues (Zingiber officinale Rosc. (Zingiberaceae) were active against phytopathogenic fungi [104].
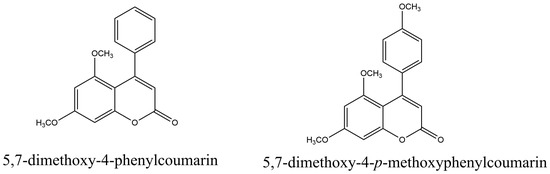
Figure 4. Naturally occurring 4-phenylcoumarins.
The naturally-occurring 3-phenylcoumarins were identified in plant species, mostly in the family Fabaceae, such as: mucodianin A from Mucuna birdwoodiana [105]; pterosonin A-F from heartwood of Pterocarpus soyauxii [106]; Sphenostylis marginata [68]; Pterocarpus soyauxii [107]; and selaginolide A from Vietnamese medicinal plant Selaginella rolandi-principis (Selaginellaceae) [108]. There is no evidence about their role in plants.
2.2.5. Pyranocoumarins
Pyranocoumarins are rare secondary metabolites of plants that contain a pyran core condensed with coumarin. These substances are distributed widely among the plant families Umbelliferae and Rutaceae. Although pyranocoumarins in the plant are very poorly studied, some studies indicate their protective role against phytopathogenic organisms [109]. However, pyranocoumarin isolated from the Rutaceae tree (Staurantus perforatus), xanthyletin, has shown significant phytotoxic effect on seed germination and root growth of Amarathus hypochondriacus (Amaranthaceae) [110]. Among the pyranocoumarins isolated from the roots of Ferulago campestris (Apiaceae), aegelinol and grandivittin have been shown to have cytotoxic properties [111]. Pyranocoumarin seselin isolated from Clausena anisata (Rutaceae) leaves acts as an antifeedant against Lucilia cuprina larvae [112].
2.2.6. Bicoumarines
Bicoumarines have been isolated from plants Triphasia trifolia (Rutaceae) [113], Dysoxylum parasiticum (Osbeck) Kosterm (Meliaceae) [114], and Pleurospermum rivulorum (Umbelliferae) [115]. The best-researched bicoumarin is dicoumarol (Figure 5). Dicumarol is generated by the hydroxylation of the 4-position of the coumarin. This is followed by capturing of a molecule of formaldehyde, and subsequently by condensation with another molecule of 4-hydroxycoumarin. Finally, the enolization of the keto group forms dicumarol [68]. Dicumarol is discovered as constituent of sweet clover hay that caused the death of cattle due to bleeding disorders. Dicoumarol is an anticoagulant that acts as a vitamin K antagonist [116]. The dicoumarol, also formed by bacterial fermentation of yellow sweet clover, was isolated for the first time from the decomposed leaves of Melilotus albus (Fabaceae/Leguminosae) [68].
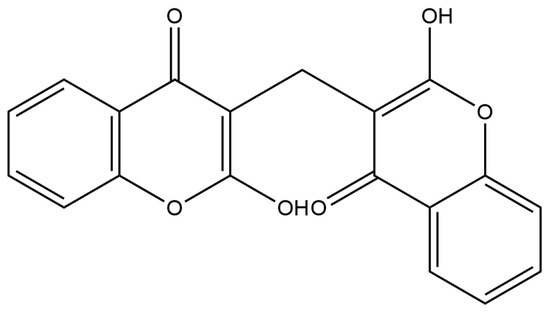
Figure 5. Structure of naturally occurring bicoumarine—dicoumarol.
The presence of natural coumarins in different species of plants and microbes, and their biological effects are summarized in Table 1. It is evident that the diversity and structural complexity of the coumarins constitute is a consequence of higher plant evolution. Simple coumarins are the most common in fungi and all angiosperms. They exhibit a wide range of biological effects related to plant protection from pathogen microbes, fungi, and nematodes. Furanocoumarin is the second most prevalent type of coumarin. Furanocoumarins are found in the family Citrus, Apiaceae, and Rutaceae, with most of them showing nematicidal properties. Pyranocoumarins are present in Apiaceace and Fabaceae, where they exhibit antifungal, phytotoxic, and antifeedant effects. Phenylcoumarins are the most abundant in the plant family Fabaceae, but there is no literature on their functions in plants.
Table 1. The presence of natural coumarins in different species of microbes, spongi and plants with their biological effects.
| Phylum/ Famillies |
Species | Group of Coumarins | Specific Compounds | Known Biological Activities | Ref. |
|---|---|---|---|---|---|
| Bacteria | |||||
| Streptomyces | Streptomyces roseochromogenes var. oscitans | 3-amino-4,7-dihydroxycoumarins | clorobiocin, novobiocin, coumermycin |
antibacterial | [15] |
| Porifera | |||||
| Axinellidae | Axinella cf. corrugate | simple | esculetin-4-carboxylic acid esters | anti-SARS-CoV | [22] |
| Fungi | |||||
| Pleosporaceae | Alternaria alternata | simple | isofraxidin | antibacterial | [61] |
| Trichocomaceae | Aspergillus fumigatus Fresenius | simple, bicoumarins | 4-hydroxycoumarin, dicoumarol | biosynthesis of coumarin | [65,66] |
| Physalacriaceae | Armillariella tabescens | simple | armillarisin A | choleretic | [73] |
| Fomitopsidaceae | Fomitopsis officinalis | simple | 6-chloro-2-oxo-4-phenyl-coumarins | anti-TBC | [74] |
| Lophiostomataceae | Lophiostoma sp. Sigrf10 | 3,4-dihydroisocoumarin | lophiostomin derivatives | antifungal, antibacterial |
[88] |
| Plants | |||||
| Citrus | C. maxima, C. medica, C. reticulata, C. micrantha | simple, furanocoumarin | unknown | [99] | |
| Citrus sinensis, C. reticulata, C. aurantifolia | simple, furanocoumarin | limettin, isopimpinellin, psoralen, bergamottin | antifungal | [100] | |
| Cucurbitaceae | Citrullus lanatus | simple | derivates of 5,7-dimethoxycoumarin | antimicrobial | [91] |
| Apiaceae | Ferulago campestris | pyranocoumarin | aegelinol, grandivittin, | cytotoxicity | [111] |
| (or Umbelliferae) | furanocoumarin | bergapten, felamidin, isoimperatorin |
antimicrobial, antioxidant |
[16] | |
| Notopterygium incisum | dihidrofuranocoumarin | columbianetin | nematicidal | [96] | |
| linear furanocoumarin | isoimperatorin | nematicidal | |||
| Petroselinum crispum | furanocoumarins | xanthotoxin, psoralen, bergapten | nematicidal | [97] | |
| Angelica pubescens Maxim. f. biserrata Shan et Yuan | simple, dihidrofuranocoumarin, | osthole, columbianadin | nematicidal | [98] | |
| furanocoumarin | bergapten, xanthotoxin | nematicidal | [115] | ||
| Pleurospermum rivulorum | bicoumarin | rivulobirins | unknown | ||
| Opopanax hispidus(Friv.) Griseb. | dihydrofuranocoumarin | 3′-isobutyryl-3′-hydroxymarmesin | unknown | [102] | |
| simple, furanocoumarin | officinalin, oreoselon, peucedanin, | unknown | |||
| Peucedanum sp. | simple, furanocoumarin | ostruthin, osthol; isoimperatorin | insecticidal | [93] | |
| dihydropyranocoumarin | xanthalin, peuarenarin | insecticidal | |||
| dihydrofuranocoumarin | athamantin, columbianadin | insecticidal | |||
| Semenovia transiliensis | furanocoumarin | Imperatorin, xanthotoxin | herbicidal | [31] | |
| Heracleum candicans Wall. | uranocoumarin | 8-geranyloxy psolaren, imperatorin, heraclenin | nematicidal | [94] | |
| Fabaceae | Melilotus officinalis | simple | dihydrocoumarin | cytotoxicity | [24] |
| bicoumarin | dicoumarol | anticoagulant | [116] | ||
| Mucuna birdwoodiana | phenylcoumarin | mucodianin A | unknown | [107] | |
| Sphenostylis marginata | phenylcoumarin | sphenostylisin A | anticancer | [68] | |
| Pterocarpus soyauxii | phenylcoumarin | pterosonins | anticancer | [106] | |
| Millettia thonningii | pyranocoumarin, furanocoumarin |
robustic acid, thonningine-C | antifungal | [20] | |
| Solanaceae | Nicotiana tabacum | simple | scopolin, scopoletin | antiviral | [79] |
| Lamiaceae | Baikal skullcap | simple | 7.8-dihydroxy-4-methylcumarin | antibacterial | [17] |
| Brassicales | Arabidopsis thaliana | simple | scopoletin | antifungal | [78] |
| Moraceae | Ficus carica | furocoumarin | bergapten, psoralen | nematicidal | [95] |
| Meliaceae | Dysoxylum parasiticum (Osbeck) Kosterm | bicoumarin | bidysoxyletine | unknown | [114] |
| Rutaceae | Triphasia trifolia | simple, furocoumarin | umbelliferone, isopimpinellin, | unknown | [113] |
| Xanthoxylum arnottianum | dihydrofuranocoumarin | xanthoarnol | antifungal | [103] | |
| Staurantus perforatus | pyranocoumarin | xanthyletin | phytotoxic | [110] | |
| Ruta angustifolia | furocoumarin, dihydrofuranocoumarin |
chalepensin, chalepin | anticancer, antiviral |
[28] | |
| Clausena anisata | pyranocoumarin | seselin | antifeedant | [112] | |
| Thymelaeaceae | Wikstroemia indica (L.) | bicoumarin | daphnoretin | antiviral, antitumor | [29] |
| Calophyllaceae | Mammea longifolia | simple | surangib B | antifungal | [80] |
This entry is adapted from the peer-reviewed paper 10.3390/app13116535
This entry is offline, you can click here to edit this entry!
