1. Introduction
A skin wound is caused by the disruption of the epidermal layer integrity [
1]. Wound healing is activated right after the skin damage, but it is not a rapid process. It consists of a complicated procedure, including a series of well-organized cellular and molecular events [
2]. Any disruption in the cascade of events causes failure in wound healing [
3].
Wound healing can be classified as primary healing and secondary healing. Primary healing is an uncomplicated healing [
4]. It occurs in aseptic wounds with smooth borders, surgical wounds for instance. Generally, it heals within 6–8 days. Secondary healing starts when the wound is disrupted with large tissue losses or complicated with infection. Granulation tissue forms, leading to scarring. It takes longer time with higher risk of infections and poor healing [
5].
1.1. Acute and Chronic Wounds
Based on the pathogenesis and consequences, skin wounds can be classified as acute and chronic wounds [
6]. An acute wound progresses through the process of a normal wound healing, resulting in wound closure without disruption. Commonly, it follows surgical incision or trauma, such as thermal wounds, abrasions, and lacerations. It heals in a timely and orderly manner [
7]. The healing of acute wound is regulated by cytokines and different growth factors. Neutrophil, macrophage, and lymphocyte migration are involved in the inflammatory phase, which lasts for days to weeks [
8]. Chronic wound is defined when a wound fails to heal in 4 weeks [
9]. Several factors and disease conditions, such as age, hormones, immune status, psychosocial issues, genetic conditions, malnutrition, infection, insufficient oxygenation or perfusion, smoking, medications, radiation, and chemotherapy, could impair the healing process and lead to persistent pro-inflammatory condition [
10]. The chronic wounds usually include vascular ulcers (venous or arterial ulcers), diabetic ulcers, pressure ulcers, and other refractory ulcers [
11].
1.2. Different Phases in Wound Healing
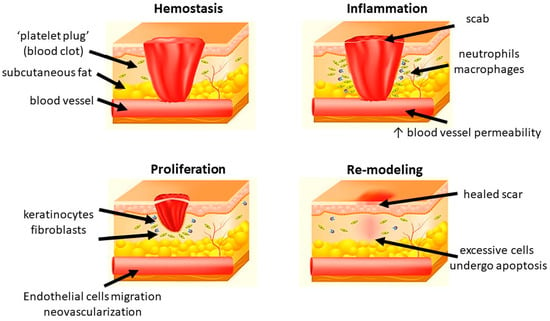
Generally, wound healing consists of four highly integrated and overlapping physiological phases, including hemostasis, inflammation, proliferation, and re-modeling. They occur in the well-organized sequence and time frame [
12]. Hemostasis is activated once the wound occurs to prevent blood loss. Subsequently, inflammatory response is activated to eliminate the foreign pathogens and to get the tissue prepare for restoration. In the proliferative phase, neovascularization, fibroblast migration, and re-epithelialization take place in a proper manner. Finally, the granulation tissue is replaced by a scar in the re-modeling phase [
13,
14,
15] (
Figure 1).
Figure 1. Different phases of wound healing.
1.2.1. Hemostasis
Hemostasis happens shortly after a wound has been formed (up to 2–4 h). Platelets reach to the wound first and form a clot to stop bleeding in a mechanical manner. After the initial “platelet plug” is formed at the site of injury, platelet-derived growth factor (PDGF) and platelet-derived SDF-1α are secreted. They contribute to the regulation of smooth muscle cells (SMCs) and bone marrow-derived endothelial progenitor cells, resulting in chemotaxis and proliferation of endothelial cells [
16,
17]. The clot also releases some cytokines, including transforming growth factor (TGF) β1, which promotes inflammatory cells proliferation and initiate the inflammatory phase. In addition to platelets, various components of blood, including certain coagulation factors and the fibrinolytic system, also play a role in this phase [
18]. Additionally, vasoconstriction leads to local hypoxia, which triggers the following oxidative stress-related cascades.
1.2.2. Inflammation
Inflammation is the body reaction against structural and functional impairments. It could be caused by mechanical forces, pathogens, toxins, and invasion of micro-organisms [
19]. Inflammation occurs soon after hemostasis (immediate up to 2–5 days) and commonly lasts for 72 h [
20]. After the vasoconstriction induced by hemostasis, the presence of histamine and other vasodilators increase the vascular permeability and therefore promote the migration of neutrophils and monocytes [
21]. Neutrophils migrate to the injury site, kill the bacteria, and clear cellular debris through generation of free radicals, secretion of proteolytic enzymes, and phagocytosis [
22,
23]. Another important regulatory cell in the inflammatory phase is macrophage which is derived from monocytes. They remove non-functional host cells, bacterial-filled neutrophils, damaged matrix, foreign debris, and remaining bacteria through phagocytic effect [
24]. Furthermore, the activated macrophages secret numerous growth factors, cytokines, and chemokines, including TGF-α, TGF-β, basic fibroblast growth factor (bFGF), PDGF, vascular endothelial growth factor (VEGF), and reactive oxygen species (ROS). They contribute to resolve inflammation, recruit endothelial cells and fibroblasts to active the proliferative phase. After this step, the levels of pro-inflammatory cytokines and oxidative stress gradually return to a basal state [
25]. In chronic wound, the healing process is stuck in the inflammatory phase, leading to pro-longed hypoxia and excessive scaring.
1.2.3. Proliferation
In this phase, epithelial proliferation is stimulated by epidermal growth factor (EGF) and TGF-α secreted by the activated platelets and macrophages [
26]. Keratinocytes are critical in the process. After injury, they will migrate across the wound bed and anchor to the cell basement membrane. The migration is activated by keratinocyte growth factor (KGF)-1, KGF-2 and IL-6 secreted by fibroblasts [
27,
28]. Once the migration completes, the keratinocytes settle, proliferate, and differentiate into epidermis [
29]. Angiogenesis is another important factor in scar formation. It is marked by endothelial cell migration and neovascularization. Endothelial cells are activated by vascular endothelial growth factor (VEGF), which is secreted by keratinocytes, macrophages, fibroblasts, and platelets. They migrate from healthy vessels and pass through the extracellular matrix (ECM) to the injury site by producing degradation factors, such as plasminogen activator and collagenase. Finally, they form new blood vessels and join the capillary loops, resulting in the provision of new blood supply to the wound. Hypoxia is reduced and ROS level decreases. After the cells are fully perfused, the excessive blood vessels undergo apoptosis [
30,
31]. Fibroblasts are essential in granulation tissue formation. They start to migrate from the wound margin in the inflammatory phase. With the stimulation of bFGF, TGF-β, and PDGF, they proliferate and synthesize glycosaminoglycans, proteoglycans, elastin, and fibronectin to form the new extracellular matrix of granulation tissue and collagen [
32]. Collagen deposition provides the tensile strength to the wound and contributes to the cell migration, proliferation, and differentiation as a feeder layer [
33]. The excessive collagen will be degraded by collagenases [
34].
1.2.4. Re-Modeling
Maturation and re-modeling are the final steps of wound healing. Clinically, re-modeling is the most important phase for patients. It is marked by the deposition and re-organization of collagen into the normal network [
35]. An abnormal re-modeling will compromise the wound strength or might form a hypertrophic scar. The whole phase may last for weeks to years. In this phase, collagen III is degraded by collagenases and replaced by collagen I [
36]. The new fibers are well-arranged into network. Meanwhile, macrophages, keratinocytes, fibroblasts, and myofibroblasts undergo apoptosis to reduce the excessive cells. The fibroblasts and matrix metallopeptidases (MMPs) regulate the ECM synthesis and cell differentiation, respectively. Taken all the above factors together, the tensile strength of the wound is enhanced, and a healed scar finally appears [
17,
37]. Meanwhile, ROS return to a physiological low level and hypoxia is abolished [
25].
2. Conventional Methods in Wound Management
The standard of wound care includes debridement, cleaning, infection management, and dressing [
38]. Debridement aims to remove the non-viable tissue and expose healthy, well-perfused tissue through surgical or autolytic/enzymatic approaches [
39]. After debridement, the wound can be cleaned using normal saline or sterile water [
40]. Detergents, hydrogen peroxide, and concentrated povidone-iodine solution are not suggested due to tissue damage and their toxicity [
41]. The management of infection is critical in wound healing. Failure in infection control leads to poor healing and abscess formation. Both topical and systemic antimicrobials are used for the infected wounds. Topical agents are commonly used for superficial wound infection, while systemic antibiotics are applied in patients with deep or systemic infection [
42]. Different types of dressing have been developed to protect the wound from infection and to promote the healing process. Dry gauze is a traditionally used wound dressing, but it may cause secondary injury during removal. Moisture-retentive dressings (MRDs) are materials with moisture vapor transmission rates (MVTRs) less than 35 g/m
2/h, which allow the wound to heal in a moist environment. There are five basic types of MRDs, including films, foams, hydrocolloids, alginates, and hydrogels. Some of them are adherent and absorbent. Some are not and need a secondary dressing to keep in place [
43].
Skin substitutes encompass a diverse range of biological, synthetic, or biosynthetic materials that can effectively provide either temporary or permanent coverage for open skin wounds. These substitutes are valuable in the treatment of both acute and chronic wounds, serving to cover defects resulting from burns or other injuries, as well as for reconstructive purposes, such as in the release of extensive post-burn contractures. They have been used in patients with wounds for a long time. The split-thickness autograft is widely used in the chronic wound. The efficiency largely depends on the quality and quantity of donor skin. The pain and donor site infection also limit the approach [
44]. Skin substrates refer to the underlying tissue supporting the skin. The bio-engineered skin substrates are a kind of dressing, which mimic the architecture of normal skin and promote wound healing in patients. They are categorized into dermal, epidermal, and full skin. According to the biologic source, the substrates can be autogenic (host of the transplant), allogenic (another human donor) or xenogenic (another species) [
45].
There are some traditional approaches, such as plant extracts and herbal medicines, used for promoting wound healing. Aloe vera is widely used in primary care. It has various biological and pharmacological activities including antioxidant, anti-inflammatory, immuno-modulatory, antimicrobial, and skin-protective [
46,
47]. NF3 is an innovative herbal formula developed by our institute. It consists of two herbs: Astragali Radix and Rehmanniae Radix in the ratio of 2:1 (
w/
w). The formula enhanced the diabetic ulcer healing through promoting angiogenesis and suppressing inflammation via in vitro, in vivo, and clinical studies [
48,
49,
50].
3. Modern Approaches in Wound Healing
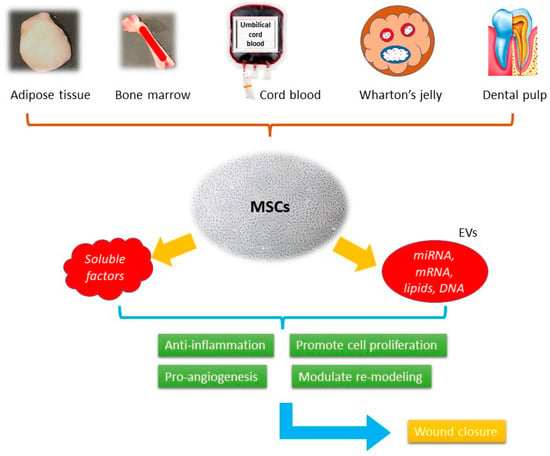
In modern therapeutic practice, the use of stem cells is becoming a promising candidate. They present with a high self-renewing capacity and various linage differentiation ability. In wound healing, they could overcome the limitations of traditional approaches, such as contamination and tissue irritation, while promoting tissue regeneration (Figure 2).
Figure 2. The schematic picture of the mechanisms of MSCs-based cell-free therapy in wound healing.
3.1. Overviews of Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells (MSCs) were first isolated from bone marrow in the 1970s, being the progenitor cells coming from mesodermal origin [
51], they could differentiate into mesenchymal lineages including osteoblasts, chondrocytes, myocytes, and adipocytes, but not hematopoietic stem cells. MSCs can be isolated from different tissues such as bone marrow, adipose tissue, peripheral blood, umbilical cords, Wharton’s jelly, and dental pulp [
52] (
Figure 1). According to the report published by International Society for Cellular Therapy (ISCT), when cultured in a standard condition (37 °C, 5% CO
2, atmospheric O
2 concentration (~20%) in a humidified incubator with media containing 10% fetal bovine serum) the minimum inclusion criteria for defining MSCs include: (i) The ability to adhere to the plastic bottom under standard culture condition; (ii) the presence of specific surface markers, such as CD73, CD105, and CD90 as well as the absence of a series of surface markers, such as CD14, CD19, CD34, and CD45; (iii) the ability to differentiate into osteoblasts, chondrocytes, and adipocytes in vitro under the effect of specific culture media [
53]. They demonstrate the therapeutic effects through immune-modulation, anti-inflammation, pro-angiogenesis, anti-oxidative, and anti-apoptotic activities [
54]. These effects are presented through paracrine activities rather than direct cell differentiation [
55]. The secretome, or the conditioned medium (CM), is a composite product secreted by MSCs. It consists of soluble proteins (mostly are growth factors, cytokine, and chemokines) and extracellular vesicles (EVs), where proteins, lipids, and genetic materials are encapsulated and transferred. Despite the availability of numerous skin substitutes in the market, stem cells are considered a better option for wound healing. They have been shown to promote healing in various types of wounds, including acute wound, chronic wound, and burn wound. It involves the use of living cells, which have the ability to actively interact with the wound environment and promote healing through the secretion of growth factors and other signaling molecules. This approach has been shown to be effective in treating chronic wounds that do not respond well to conventional therapies. When using cell-free products, such as growth factors, cytokines, and extracellular vesicles, it can stimulate the body’s natural healing mechanisms without the need for live cells [
56]. In contrast, many skin substitutes in the market are often composed of synthetic materials that lack the biological complexity and versatility of living cells.
3.2. Soluble Factors in Mesenchymal Stem Cells (MSCs) Derived Secretome
A growing number of studies demonstrated MSCs could secrete a panel of trophic factors, including cytokines, growth factors, and chemokines. The activity of the secretion, which was first described in 2006, makes MSCs a paracrine tool [
56,
57]. These trophic factors could contribute to different wound-healing phases (
Table 1).
Table 1. Proteins related to wound healing in MSCs-derived secretome [
58,
59,
60,
61,
62,
63,
64].
|
Proteins
|
Inflammation
|
Proliferation
|
Angiogenesis
|
Re-Modeling
|
Ref.
|
|
ANG-1
|
|
|
✓
|
|
[61]
|
|
BMP
|
|
✓
|
|
|
[64]
|
|
CCL2
|
✓
|
|
|
|
[62]
|
|
CCL5
|
✓
|
|
|
|
[64]
|
|
CCL20
|
✓
|
|
|
|
[62]
|
|
EGF
|
|
✓
|
✓
|
|
[63,64]
|
|
FGF-7
|
|
✓
|
|
✓
|
[60,61,64]
|
|
Follistatin
|
|
|
|
✓
|
[61]
|
|
G-CSF
|
|
✓
|
|
|
[64]
|
|
GM-CSF
|
|
✓
|
|
|
[64]
|
|
HGF
|
|
✓
|
✓
|
✓
|
[60,61,63,64]
|
|
ICAM
|
|
|
|
✓
|
[62]
|
|
IDO
|
✓
|
|
|
|
[64]
|
|
IGF
|
|
✓
|
✓
|
|
[63,64]
|
|
IL-6
|
✓
|
|
✓
|
✓
|
[60,61,63,64]
|
|
IL-8
|
✓
|
|
|
|
[63,64]
|
|
IL-10
|
✓
|
|
|
✓
|
[64]
|
|
Insulin
|
|
|
✓
|
|
[61]
|
|
LIF
|
✓
|
|
|
|
[62,64]
|
|
Lipocalin-2
|
✓
|
|
|
|
[62]
|
|
MCP-1
|
✓
|
|
✓
|
|
[60,63,64]
|
|
MMP-1
|
|
|
✓
|
✓
|
[60,64]
|
|
MMP-2
|
✓
|
|
✓
|
|
[62,64]
|
|
MMP-3
|
|
|
✓
|
✓
|
[62,64]
|
|
MMP-7
|
|
|
✓
|
✓
|
[64]
|
|
PDGF
|
|
✓
|
✓
|
|
[63,64]
|
|
PGE2
|
✓
|
✓
|
|
|
[64]
|
|
Serpin E1
|
|
|
|
✓
|
[62]
|
|
TGF-β1
|
✓
|
✓
|
✓
|
✓
|
[61,63]
|
|
TIMP-1
|
|
|
|
✓
|
[60,64]
|
|
TIMP-2
|
|
|
|
✓
|
[62,64]
|
|
uPAR
|
|
|
✓
|
✓
|
[61]
|
|
VEGF
|
|
|
✓
|
|
[58,60,61,62,63,64]
|
3.3. Extracellular Vesicles (EVs) in Mesenchymal Stem Cells (MSCs) Derived Secretome
Extracellular vesicles (EVs) carry mRNA, mircro RNA, and proteins to modulate intercellular communications. They can be found in almost all biological fluids. Based on the size of EVs, they could be categorized into three fractions: exosomes (Exos), microvesicles (MVs), and apoptotic bodies [
71]. Exosomes (Exos), with the size of 20 and 150 nm, are formed within the endosomal space. They are released into the extracellular area after the fusion with the multivesicular bodies of the plasma membrane. Exos were first reported in 1983 as vesicles contributing to mammalian reticulocyte differentiation and maturation [
72]. In the recent research, they were found to be similar to MSCs, and played an important role in cell–cell communication [
73]. Microvesicles (MVs), with a larger size of 100 to 1000 nm, are formed by direct budding of the plasma membrane. Apoptotic bodies, with a diameter between 50 nm and 5 µm, are secreted by membrane blebbing as programmed cell death occurs [
74]. Since the size of different fractions could be overlapping, the general term “extracellular vesicles (EVs)” will be used in the following section. The International Society for Extracellular Vesicles (ISEV) has published the latest Minimal Information for Studies of Extracellular Vesicles (MISEV) guidelines, which outline the criteria that should be followed for each preparation of EVs. These criteria include quantitative measures of the source of EVs (such as the number of secreting cells, volume of biofluid, or mass of tissue), determination of EV abundance (total particle number and/or protein or lipid content), identification of components associated with EV subtypes or EVs generically, and identification of any non-vesicular, co-isolated components [
74]. Differential ultracentrifugation is the most commonly used technique for separating EVs. However, other chemical-based techniques, such as density gradients, precipitation, and immune-isolation, are also available. The choice of EV isolation method depends on various factors, including the desired recovery rate, specificity, and purity. The therapeutic effects of MSCs-derived EVs are related to their cargo, including microRNA, mRNA, and proteins, and have been demonstrated in the wound models (
Table 2) [
75,
76,
77,
78,
79,
80,
81,
82,
83,
84,
85,
86,
87,
88,
89,
90,
91].
Table 2. Contents identified in MSCs-EVs related to wound healing [
76,
77,
78,
79,
80,
81,
82,
83,
84,
85,
86,
87,
88,
89,
90,
91].
|
Inflammation
Refs. [76,77,78,79,80,81,82]
|
Proliferation
Refs. [84,85,86,87,88]
|
Angiogenesis
Refs. [90,91]
|
Re-modeling
Refs. [92,93]
|
|
miR-223, miR-let7b, miR-181c, miR-let7, miR-182, miR-155
|
Wnt-4, miR-21, miR-378, miR-19b, MALAT1
|
miR-135b-5p, miR-499a-3p, miR-126
|
Wnt-4, miR-21, miR-23a, miR-125b, miR-145, miR-29a-3p
|
3.4. Clinical Trials Using MSCs-Based Cell-Free Products
The therapeutic effects of MSC-derived secretome have been demonstrated in various disease models. Nevertheless, their clinical application remains limited. According to the records on a database of funded clinical studies conducted around the world (
www.clinicaltrials.gov (accessed on 13 March 2023), there is one completed phase I clinical trial registered, in which conditioned medium from WJ-MSC has been used for chronic ulcer (NCT04134676). A total of 38 patients with chronic ulcer for more than one month were enrolled, and topical conditioned medium gel covered by transparent dressing was applied for two weeks. The evaluation and dressing replacement were performed once a week. The primary results suggested that edema and erythema were reduced, granulation tissue presented, and the size of ulcer reduced. In terms of exosome, there is also one completed phase I clinical trial registered (NCT02565264). About 5 participants were enrolled. Autologous plasma-derived exosome was applied to the ulcer daily for 28 days. Ulcer size and pain level were recorded once a week. Unfortunately, neither results nor related publications have been uploaded by the investigators so far.
Except for wound management, there are some clinical trials registered using MSC-based cell free products for other diseases, such as Alzheimer’s disease (NCT04388982), stroke (NCT05008588), alopecia (NCT05658094, NCT05296863), and dry eye diseases (NCT04213248, NCT05738629). Since the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 2019, a great number of clinical trials have been registered using MSCs-derived CM (secretome) or exosomes against the respiratory symptoms.
3.5. Hopes and Challenges
3.5.1. Advantages of MSC-Based Cell-Free Therapy
Although the use the cell-based products to promote wound healing is not new, there are some barriers to overcome. Most of the commercial cell-based skin graft substitutes, such as Apligraf
®, Recell
®, PolyActive
®, and OrCel
®, are expensive [
95]. They require special and strict conditions for storage and transportation. Furthermore, there are potential risks, including tumorgenicity, rejection, and infection, make them difficult to be used commonly. The delivery of living cells to the wound site is another challenge. The injection of cells through a needle will damage cell membrane and viability [
96]. After injection, the excessive apoptotic or necrotic cells may elicit the local immune response, leading to a secondary impairment. Hence, different acellular approaches are needed.
Numerous preclinical studies have demonstrated the therapeutic effects of MSCs-derived cell-free products (MSCs-CM). Different animal models were used to investigate their efficacy. The in vitro experiments contributed to a better understanding of the underlying mechanisms. Compared with the cell-based products, the MSCs-CM can be manufactured, packaged, stored, and transported more easily. Large-scale production is therefore possible. Treated as pharmacological agents, the quality control of the cell-free products is simpler. There is no need to match the donor and the recipient and can avoid the immune rejection. Furthermore, they can reduce the possibility of emboli formation, tumorgenicity, and infection transmission [
97]. Therefore, unlike cell-based products, MSCs-CM can be regarded as a ready-to-use pharmacological agent. In general, isolated cell-free products can be stored at temperatures ranging from −20 °C to −80 °C for up to 6 to 7 months [
98].
3.5.2. Constraints and Challenges in Cell-Free Products
Although the effectiveness of MSC-based cell-free products have been demonstrated in many preclinical studies, there are still limited clinical research and commercial products. The first challenge is the profiling of the components. In addition to the soluble factors, there are different EVs in secretome, containing miRNAs, lipids, and long noncoding RNAs. Their identification and bioactivities are not completely understood. The comprehensive and quantitative analysis of the biomolecules in secretome using “omics” approaches is needed to clarify their profile and functions. The lack of a standardized isolation and cultivation protocol is the second challenge in the field. The difference in donors (gender, age, tissue source), cell passage and numbers (seeding density), laboratory conditions (medium, flask, oxygen tension), and preparation procedure (particularly ultracentrifugation versus precipitation for EVs) lead to the variation in the components of secretome from batch to batch. As an illustration, the secretome profile of MSCs derived from varying tissue sources can differ, while the composition of the medium and culture supplements used can affect cell viability and proliferative capacity. Additionally, studies have revealed that subjecting cells to lower oxygen tension (below 5%) can enhance the regenerative potential of the secretome, particularly in the context of ischemic diseases. The production of MSC-secretome using pharmacological standard could reduce the inconsistency. Compliance with a good manufacturing practice (GMP) standard protocol is also necessary to translate the cell-free products bench to bed [
63,
99]. Up to today, there are not enough comprehensive studies focusing on the different application protocols for secretome, such as preparation methods, concentrations, and delivery routes. A specific protocol may be needed for a given pathology. Meanwhile, in most reported studies, fetal bovine serum (FBS) was used for MSCs’ cultivation. It could be unsuitable for clinical use due to possible disease transmission and xenogeneic immune reaction [
100]. Therefore, human serum or knockout serum replacement is needed, which will largely increase the cost of the cell-free products as expected [
101].
4. Conclusions
MSCs-based cell-free products have emerged as a new and promising therapeutic option in tissue regeneration. They bypass the risks of tumorgenicity, immune rejection, and ethical issues in cell therapy. A growing number of preclinical studies have demonstrated the therapeutic effects of MSCs secretome, including the soluble factors and EVs. However, the number of clinical trials remains limited. To get a large-scale clinical grade cell-free product, a well-defined and standardized manufacturing protocol is needed. The experiences in developing GMP compliant pharmacological agents can be applied. Despite the incomplete knowledge about the underlying mechanisms, the cell-free therapy provides an additional therapeutic option for patients suffering from chronic non-healing wound.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24119356