Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Patients with cardiovascular disease (CVD) and chronic kidney disease (CKD) show high rates of cardiorenal outcomes. Several studies suggest that the activation of the mineralocorticoid receptor (MR) induces cardiac and renal injury, including inflammation and fibrosis. Finerenone is a novel non-steroidal selective mineralocorticoid receptor antagonist (MRA) with a stronger mineralocorticoid receptor binding potential than eplerenone and spironolactone. Finerenone has been shown to have anti-inflammatory and anti-fibrotic effects in preclinical studies.
- chronic kidney disease
- cardiovascular disease
- type 2 diabetes
- mineralocorticoid receptor
- mineralocorticoid receptor antagonist
- finerenone
1. Introduction
Cardiovascular disease (CVD) and chronic kidney disease (CKD) are closely connected since they share common risk factors and pathophysiological pathways and influence mutual evolution. Indeed, a reduced cardiac performance, such as in a heart failure (HF) setting, affects renal functions through an activated neurohormonal and inflammatory cascade, and increases the venous pressure and hypoperfusion of the kidneys. In contrast, CKD impacts cardiovascular functions by inducing hypertension and vascular calcification [1,2,3]. In patients with CKD requiring dialysis, CVD is recognized as the leading cause of death [4], whereas CKD is an independent predictor of mortality and morbidity in patients with HF [5].
Therefore, the turning point in the prognosis and long-term management of CVD and CKD may be the early use of drugs that act simultaneously on the heart and kidney. Particularly, a treatment with sacubitril/valsartan leads to a slower decline in renal functions and improves cardiovascular outcomes in patients with HF [6]. However, the optimal titration of this combination is often hampered by suboptimal creatinine levels, as only few studies included patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min [7]. Similarly, the use of sodium–glucose transporter 2 inhibitors (SGLT2i) for the treatment of heart failure also offers renal protection [8,9]. A recent study in patients with advanced CKD (eGFR 25–45 mL/min) showed that empagliflozin was associated with a reduced risk of renal progression or cardiovascular death compared to a placebo (hazard ratio, 0.72; 95% confidence interval [CI], 0.64–0.82; p < 0.001) [10]. Despite this, cardiovascular outcomes are generally worse in patients with CKD than in those with normal renal functions. It should be noted that patients with impaired renal functions cannot benefit from optimized cardiovascular medical therapy, as there are no studies supporting its prescription in this setting.
The renin-angiotensin-aldosterone system (RAAS) is the direct link between the heart and the kidneys and is one of the main mechanisms for the homeostasis of sodium, volume, osmolarity, renal blood flow, and blood pressure [11]. Studies have shown that the activation of the mineralocorticoid receptor (MR) induces cardiac and renal injury, including inflammation and fibrosis [12,13]. In this context, mineralocorticoid receptor antagonists (MRAs) were developed and are now widely used in the treatment of HF, refractory hypertension, and various renal diseases [5]. They work by inhibiting the action of aldosterone on its receptor. This results in a reduction in cardiac remodeling, a reduction in inflammation, and a reduction in proteinuria [14,15]. Finerenone is a novel non-steroidal selective MRA with a stronger mineralocorticoid receptor binding potential than eplerenone and spironolactone [12,16]. Finerenone has been shown to have anti-inflammatory and anti-fibrotic effects in preclinical studies [16]. It has also been shown to improve cardiorenal outcomes in patients with type 2 diabetes mellitus (DM) and mild to severe CKD (FI-DELIO-DKD and FIGARO-DKD clinical trials) [17,18].
2. Finerenone: Biochemical Characteristics and Experimental Studies
Finerenone belongs to the class of selective nonsteroidal MRAs, exhibiting a higher binding affinity to the mineralocorticoid receptor compared to eplerenone and spironolactone, as well as showing chemical and physical properties that provide a more balanced cardio–renal drug delivery (Figure 1) [16]. Drug metabolism is pre-dominantly hepatic, involving cytochrome P450 3A4 (CYP3A4) and cytochrome P450 2C8 (CYP2C8) [61]. Finerenone could be an inhibitor of CYP2C8 (reversible) and CYP3A4 (reversible and irreversible) and an inducer of CYP3A4 with a 20 mg daily dose, as demonstrated by in vitro experiments with hepatic microsomes and human hepatocytes [62,63]. However, recent studies investigating the once-daily 20 mg administration showed no significant drug–drug interactions with the cytochrome P450 enzyme substrates [64].
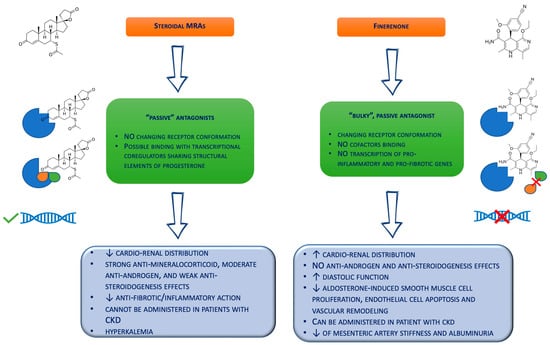
Figure 1. Biochemical differences between the steroidal mineralocorticoid receptor antagonists and finerenone. CKD = chronic kidney disease; MRAs = mineralocorticoid receptor antagonists.
Specifically, although a potential clinically relevant induction of CYP3A4 was shown in in vitro experiments, a weak induction was confirmed in vivo [62,64]. In addition, the renal function also influenced the drug clearance. As kidney dysfunction worsened and the clearance decreased, a longer elimination half-life was observed, albeit with no affection on the maximum serum concentration [61]. Furthermore, finerenone shows greater polarity, defined as a higher polar surface area, and is less lipophilic compared to steroidal MRAs [65]. Indeed, finerenone does not cross the blood–brain barrier and preclinical studies demonstrated how this drug was not detected in brain tissue after oral administration [66]. Finally, in hypo-albuminemia, an elevated blood concentration of the drug is observed, as the levels of finerenone are influenced by the serum albumin, which is the main binding protein of this drug [67].
Notably, molecular studies suggest that finerenone acts on the mineralocorticoid receptors as a “bulky” passive antagonist, different from steroidal MRAs [68]. Specifically, this agent works as a bulky substituent of the MR, changing the receptor conformation to avoid binding with cofactors and the transcription of pro-inflammatory and pro-fibrotic genes [69]. Thus, this non-steroidal MRA is able to reduce the MR nuclear accumulation, inhibit the receptor recruitment onto DNA target sequences, and suppress recycling and the induction of mutant forms of the mineralocorticoid receptor [69]. Moreover, finerenone demonstrated a balanced distribution in the cardiac and renal tissues of mice [16], compared to eplerenone and spironolactone, which revealed higher concentrations in the kidney rather than the heart [16]. This combination of the potency and selectivity on the MR and the cardio–renal distribution could translate into expanded protection on the kidneys and heart, particularly in high-risk patients with altered renal functions. In fact, the half-life of finerenone is not affected by mild renal impairment, while it is slightly increased in patients with moderate and severe renal impairment. [61]. However, renal impairment showed no significant effect on the maximum plasma concentration [61]. Moreover, finerenone demonstrated a short half-life in patients with renal failure (about 3 h) and no active metabolites [63]. Conversely, spironolactone metabolites could be measured in approx. 40% of patients with an eGFR of 25–45 mL/min/1.73 m2 for up to 3 weeks after stopping the drug administration [70].
Firstly, Bonnard et al. showed that in mice with CKD induced using subtotal nephrectomy, treatment with finerenone improved the diastolic function, which was severely impaired due to cardiac hypertrophy and fibrosis [71]. Finerenone also reduced the plasmatic levels of the prohormone of the brain natriuretic peptide [16].
Beyond its benefits on cardiac remodeling, finerenone showed favorable effects on the vascular system. This agent dose-dependently reduced the aldosterone-induced smooth muscle cell proliferation and endothelial cells apoptosis [72]. Furthermore, it was able to accelerate re-endothelialization and inhibit vascular remodeling after vascular damage [72]. In addition, in mice with predefined CKD, finerenone could improve endothelial dysfunction through an increased nitric oxide (NO) bioavailability and reduced levels of superoxide anion levels [73], thus decreasing the oxidative stress and its negative effects on the cardiovascular system [15]. Interestingly, the administration of finerenone improved metabolic syndrome-related disorders, such as diastolic cardiac dysfunction and nephropathy, in rats with metabolic syndromes. This benefit was mediated by short-term effects, such as improving myocardial perfusion and decreasing the oxidative stress, and long-term effects, such as reducing the LV diameters and LV end-diastolic pressure [74]. Moreover, finerenone was associated with reduced mesenteric artery stiffness and albuminuria in animal models, which were directly related to CKD and increased cardiovascular morbidity and mortality [75]. Furthermore, as shown by Kolkhof et al. [16], finerenone could induce end-organ protection with a decreased risk of electrolyte disturbances.
3. Finerenone: From Bench to Bedside
Clinical trials which evaluated the role of finerenone on cardiovascular outcomes were recently published, and several are still ongoing [68].
In the phase II Mineralocorticoid Receptor Antagonist Tolerability Study (ARTS) trial, the authors demonstrated a similar efficacy of the daily finerenone treatment (5 or 10 mg) compared to spironolactone in patients suffering from HF with a reduced ejection fraction and mild CKD and with lower increases in the serum potassium levels and slight reduction in the eGFR [66]. Specifically, the administration of finerenone was associated with significantly smaller mean increases in the potassium levels compared to spironolactone (0.04–0.30 and 0.45 mmol/L, respectively, p < 0.0001–0.0107) and reduced rates of hyperkalemia (5.3 and 12.7%, respectively, p = 0.048) [66].
Additionally, the phase IIb ARTS-Diabetic Nephropathy (ARTS-DN) trial showed the efficacy and safety of finerenone in patients with DM and high or very high proteinuria due to an improvement in the urinary albumin–creatinine ratio with no difference in the rate of hyperkalemia and the eGFR reduction compared to the placebo [76]. Concordantly, the Mineralocorticoid Receptor Antagonist Tolerability Study-Heart Failure (ARTS-HF) was designed to compare the efficacy and safety of finerenone with eplerenone in patients with type 2 DM and/or CKD suffering from chronic HF with a reduced ejection fraction and already treated with evidence-based therapy for HF for at least three months [77]. Specifically, the study design consisted of five pre-planned finerenone treatment arms and one current eplerenone treatment. The primary aim was to examinate the efficacy and safety of different oral doses of finerenone given once per day [77]. While the primary endpoint of a 30% reduction in NT-proBNP level after 90 days was not achieved (with similar results in the two groups), the incidence of the exploratory composite endpoint of death from any cause, cardiovascular hospitalization, or emergency presentation for worsening HF at day 90 was significantly lower in most finerenone groups (especially the 10 titrated up to 20 mg dose group) compared to eplerenone [77]. Notably, an increase in the serum potassium levels above ≥5.6 mmol/L at any time was observed in approx. 4% of patients, with a similar distribution among all the treatment groups [77].
The two largest trials that explored the clinical benefit of finerenone on the renal and cardiovascular outcomes in patients with mild to severe CKD and type 2 DM, on top of the maximum tolerated renin–angiotensin system inhibition, were the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) [17] and the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD) trials [18].
FIDELIO-DKD was a double-blind, placebo-controlled trial in which the patients were randomized 1:1 to finerenone (10 or 20 mg) or placebo treatments. Different from the previous studies, the patients with previous HF with a reduced ejection fraction were excluded from the study [17]. Chronic kidney disease was defined as either persistent, moderately increased albuminuria (urinary albumin-to-creatinine ratio [UACR] ≥30–<300 mg/g) with an eGFR of ≥25–<60 mL/min/1.73 m2 and the presence of diabetic retinopathy, or persistent, severely increased albuminuria (UACR ≥300–≤5000 mg/g) with an eGFR of ≥25–<75 mL/min/1.73 m2 [17]. The cardiovascular outcome was a composite of the time to the first onset of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for HF. The kidney outcome was a composite of kidney failure (defined as chronic dialysis for >90 days, kidney transplantation, or eGFR <15 mL per min per 1.73 m2), a sustained ≥40% decrease in the eGFR from the baseline over at least four weeks, or renal death [17]. Otherwise, the secondary cardiac outcome consisted of a composite of death from CV causes, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for HF, with a time-to-event analysis. The results demonstrated a lower incidence of the composite cardiovascular outcome in the finerenone group than in the placebo group (367 [13.0%] and 420 [14.8%] patients, respectively; [95% CI, 0.75–0.99]; p = 0.034) regardless of the presence of previous CVD.
A lower incidence of the composite renal outcome was also achieved in the finerenone-treated group compared to the placebo (200 [15.3%] vs. 267 [20.5%], respectively), particularly in the patients with CVD [17]. In addition, Filippatos et al. published a secondary analysis of the FIDELIO-DKD trial analyzing the effects of the novel MRA on the incidence of new onset atrial fibrillation/atrial flutter in the same cohort of patients [78]. Interestingly, in the group of patients randomized for the finerenone treatment, there was a reduction in the absolute risk of new onset atrial arrhythmias and a lower incidence of renal or cardiovascular events regardless of a history of atrial fibrillation/flutter at the baseline [78].
The FIGARO-DKD was a randomized, double-blind, placebo-controlled study where patients with type 2 diabetes and CKD were randomly assigned for finerenone treatment or placebo [18]. Chronic kidney disease in FIGARO-DKD was defined as either persistent, moderately increased albuminuria (UACR ≥ 30–<300 mg/g) with an eGFR of ≥25–≤90 mL/min/1.73 m2, or persistent, severely increased albuminuria (UACR ≥ 300–≤ 5000 mg/g) with an eGFR ≥ 60 mL/min/1.73 m2 [18]. The primary outcome was a composite of death from CV causes, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for HF. The secondary outcome was a composite of kidney failure (defined as chronic dialysis for >90 days, kidney transplantation, or eGFR < 15 mL per min per 1.73 m2 for at least four weeks), a sustained decrease from the baseline of at least 40% in the eGFR, or death from renal causes [18]. The primary outcome occurred in 12.4% of the patients treated with the novel MRA, compared to 14.2% in the placebo group (p = 0.03). The secondary outcome occurred in 9.5% of the treatment group vs. 10.8% of the placebo-treated patients [18]. Remarkably, this study demonstrated a significant reduction in cardiovascular outcome in the finerenone group, with the major contribution driven by a lower incidence of hospitalization and a reduction in the secondary kidney outcome, albeit not significant [18]. Interestingly, a study on the combined effect of finerenone and empagliflozin in patients with type 2 diabetes and CKD is still ongoing and is estimated to be completed by 2024 [79]. This study is designed to evaluate the cumulative efficacy, safety, and tolerability of dual therapy, including finerenone and empagliflozin in people with CKD and diabetes [79]. Considering the well-known protective effects of sodium–glucose transporter 2 inhibitors (SGLT2i) on the cardiovascular system [80] and renal functions [9], dual therapy with finerenone and SGLT2i could provide an additive effect in order to slow the disease progression and provide long-term benefits for patients with diabetes and CKD [79].
Based on the above trials, both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approved the marketing of finerenone in routine clinical care for patients with CKD and DM, although studies are currently ongoing to formulate economic models to evaluate its use. Indeed, drug utilization in the real world is unequal from nation to nation and is influenced by drug reimbursability ($22.13 per unit in USA), local care factors, and standard medical therapy. The ongoing FINE REAL study aims to evaluate the use of finerenone in a variety of states by relating it to specific routine clinical care, and by assessing its impact in terms of its improvement in the progression of microvascular complications and renal functions [81].
This entry is adapted from the peer-reviewed paper 10.3390/jcdd10060236
This entry is offline, you can click here to edit this entry!
