Coronavirus infections are neuroinvasive and can provoke injury to the central nervous system (CNS) and long-term illness consequences. They may be associated with inflammatory processes due to cellular oxidative stress and an imbalanced antioxidant system. The ability of phytochemicals with antioxidant and anti-inflammatory activities, such as Ginkgo biloba, to alleviate neurological complications and brain tissue damage has attracted strong ongoing interest in the neurotherapeutic management of long COVID. Ginkgo biloba leaf extract (EGb) contains several bioactive ingredients, e.g., bilobalide, quercetin, ginkgolides A–C, kaempferol, isorhamnetin, and luteolin. They have various pharmacological and medicinal effects, including memory and cognitive improvement. Ginkgo biloba, through its anti-apoptotic, antioxidant, and anti-inflammatory activities, impacts cognitive function and other illness conditions like those in long COVID.
- Ginkgo biloba bioactive compounds
- neuroinvasive coronavirus infection
- neurological long COVID
1. Introduction
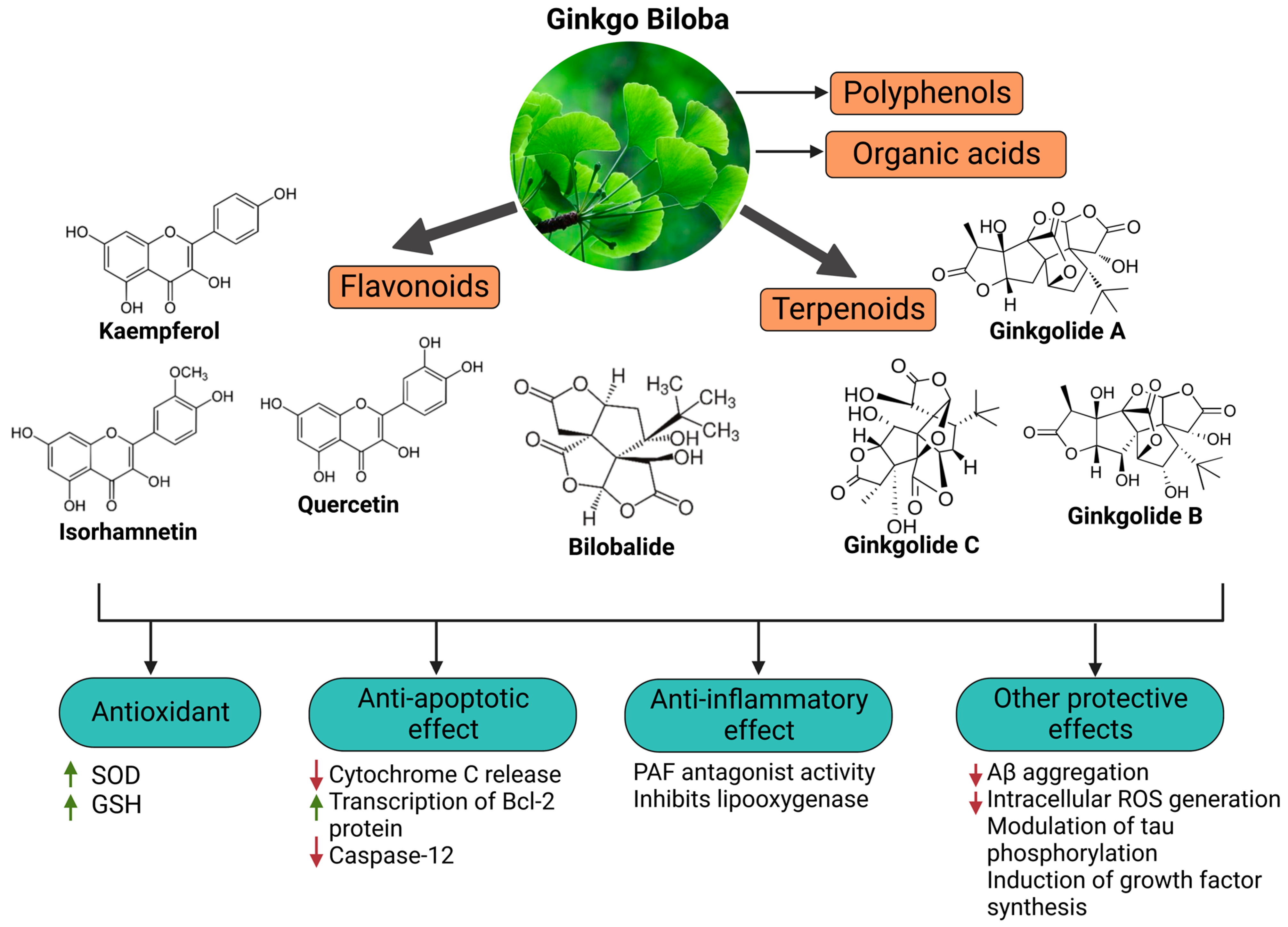
2. Long-Term Neurological Damage and the Role of Oxidative Stress
2.1. Oxidative Stress and Redox Signaling, Players in SARS-CoV-2 Neurological Damage
2.2. In Vivo and In Vitro Models of Oxidative Stress
3. Ginkgo Biloba Extract (EGb) for Neuroprotection and Potential Regeneration from Long COVID Syndrome
3.1. Ginkgo Biloba Antioxidative and Anti-Inflammatory Effects
3.2. Neuroprotective, Anti-Apoptotic, and Anxiolytic Drug Effects of EGb
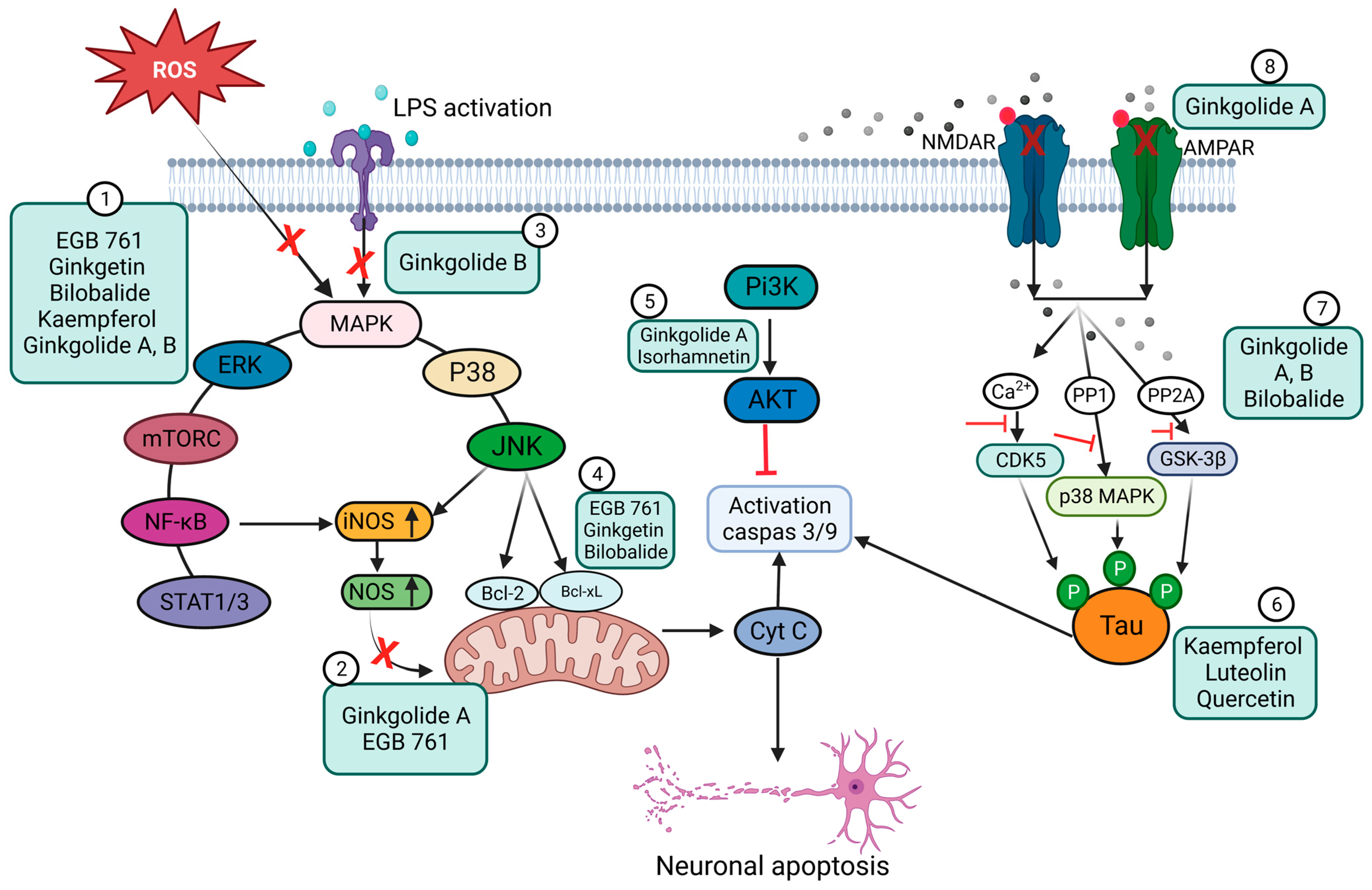
4. Ginkgo-Biloba-Based Nanotherapy for Neuroprotection and Regeneration from SARS-CoV-2 Neurological Damage
5. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15051562
References
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An Overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 869–875.
- Wieczfinska, J.; Kleniewska, P.; Pawliczak, R. Oxidative Stress-Related Mechanisms in SARS-CoV-2 Infections. Oxidative Med. Cell. Longev. 2022, 2022, 5589089.
- Kase, Y.; Okano, H. Neurological Pathogenesis of SARS-CoV-2 (COVID-19): From Virological Features to Clinical Symptoms. Inflamm. Regen. 2021, 41, 15.
- Nuzzo, D.; Picone, P. Potential Neurological Effects of Severe COVID-19 Infection. Neurosci. Res. 2020, 158, 1–5.
- Edinoff, A.N.; Chappidi, M.; Alpaugh, E.S.; Turbeville, B.C.; Falgoust, E.P.; Cornett, E.M.; Murnane, K.S.; Kaye, A.M.; Kaye, A.D. Neurological and Psychiatric Symptoms of COVID-19: A Narrative Review. Psychiatry Int. 2022, 3, 158–168.
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What Is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119.
- Ahamed, J.; Laurence, J. Long COVID Endotheliopathy: Hypothesized Mechanisms and Potential Therapeutic Approaches. J. Clin. Investig. 2022, 132, e161167.
- Carfì, A.; Bernabei, R.; Landi, F.; for the Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603.
- Pilotto, A.; Cristillo, V.; Cotti Piccinelli, S.; Zoppi, N.; Bonzi, G.; Sattin, D.; Schiavolin, S.; Raggi, A.; Canale, A.; Gipponi, S.; et al. Long-Term Neurological Manifestations of COVID-19: Prevalence and Predictive Factors. Neurol. Sci. 2021, 42, 4903–4907
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome. Lancet Respir. Med. 2020, 8, 420–422
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474.
- Villa, C.; Rivellini, E.; Lavitrano, M.; Combi, R. Can SARS-CoV-2 Infection Exacerbate Alzheimer’s Disease? An Overview of Shared Risk Factors and Pathogenetic Mechanisms. JPM 2022, 12, 29.
- Domingues, R.B.; Mendes-Correa, M.C.; de Moura Leite, F.B.V.; Sabino, E.C.; Salarini, D.Z.; Claro, I.; Santos, D.W.; de Jesus, J.G.; Ferreira, N.E.; Romano, C.M.; et al. First Case of SARS-COV-2 Sequencing in Cerebrospinal Fluid of a Patient with Suspected Demyelinating Disease. J. Neurol. 2020, 267, 3154–3156
- Xiong, Y.; Zhu, G.-H.; Wang, H.-N.; Hu, Q.; Chen, L.-L.; Guan, X.-Q.; Li, H.-L.; Chen, H.-Z.; Tang, H.; Ge, G.-B. Discovery of Naturally Occurring Inhibitors against SARS-CoV-2 3CLpro from Ginkgo Biloba Leaves via Large-Scale Screening. Fitoterapia 2021, 152, 104909.
- Chen, Z.; Cui, Q.; Cooper, L.; Zhang, P.; Lee, H.; Chen, Z.; Wang, Y.; Liu, X.; Rong, L.; Du, R. Ginkgolic Acid and Anacardic Acid Are Specific Covalent Inhibitors of SARS-CoV-2 Cysteine Proteases. Cell Biosci. 2021, 11, 45.
- Sochocka, M.; Ochnik, M.; Sobczyński, M.; Gębura, K.; Zambrowicz, A.; Naporowski, P.; Leszek, J. Ginkgo Biloba Leaf Extract Improves an Innate Immune Response of Peripheral Blood Leukocytes of Alzheimer’s Disease Patients. Nutrients 2022, 14, 2022
- Barbalho, S.M.; Direito, R.; Laurindo, L.F.; Marton, L.T.; Guiguer, E.L.; Goulart, R.D.A.; Tofano, R.J.; Carvalho, A.C.A.; Flato, U.A.P.; Capelluppi Tofano, V.A.; et al. Ginkgo Biloba in the Aging Process: A Narrative Review. Antioxidants 2022, 11, 525.
- Tomino, C.; Ilari, S.; Solfrizzi, V.; Malafoglia, V.; Zilio, G.; Russo, P.; Proietti, S.; Marcolongo, F.; Scapagnini, G.; Muscoli, C.; et al. Mild Cognitive Impairment and Mild Dementia: The Role of Ginkgo Biloba (EGb 761®). Pharmaceuticals 2021, 14, 305.
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and Antioxidant Effect of Ginkgo Biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics 2019, 16, 666–674.
- Al-kuraishy, H.M.; Al-Gareeb, A.I.; Kaushik, A.; Kujawska, M.; Batiha, G.E. Ginkgo Biloba in the Management of the COVID-19 Severity. Arch. Pharm. 2022, 355, 2200188.
- Sulzer, D.; Antonini, A.; Leta, V.; Nordvig, A.; Smeyne, R.J.; Goldman, J.E.; Al-Dalahmah, O.; Zecca, L.; Sette, A.; Bubacco, L.; et al. COVID-19 and Possible Links with Parkinson’s Disease and Parkinsonism: From Bench to Bedside. NPJ Park. Dis. 2020, 6, 18.
- Chaudhry, Z.; Klenja, D.; Janjua, N.; Cami-Kobeci, G.; Ahmed, B. COVID-19 and Parkinson’s Disease: Shared Inflammatory Pathways Under Oxidative Stress. Brain Sci. 2020, 10, 807.
- Serrano-Castro, P.J.; Estivill-Torrús, G.; Cabezudo-García, P.; Reyes-Bueno, J.A.; Ciano Petersen, N.; Aguilar-Castillo, M.J.; Suárez-Pérez, J.; Jiménez-Hernández, M.D.; Moya-Molina, M.Á.; Oliver-Martos, B.; et al. Impact of SARS-CoV-2 Infection on Neurodegenerative and Neuropsychiatric Diseases: A Delayed Pandemic? Neurología 2020, 35, 245–251.
- Cavezzi, A.; Troiani, E.; Corrao, S. COVID-19: Hemoglobin, Iron, and Hypoxia beyond Inflammation. A Narrative Review. Clin. Pract. 2020, 10, 1271
- Idrees, D.; Kumar, V. SARS-CoV-2 Spike Protein Interactions with Amyloidogenic Proteins: Potential Clues to Neurodegeneration. Biochem. Biophys. Res. Commun. 2021, 554, 94–98.
- Cavezzi, A.; Troiani, E.; Corrao, S. COVID-19: Hemoglobin, Iron, and Hypoxia beyond Inflammation. A Narrative Review. Clin. Pract. 2020, 10, 1271.
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Pedraza-Chaverri, J.; Scholze, A. Nrf2 Activation in Chronic Kidney Disease: Promises and Pitfalls. Antioxidants 2022, 11, 1112.
- Horie, Y.; Suzuki, T.; Inoue, J.; Iso, T.; Wells, G.; Moore, T.W.; Mizushima, T.; Dinkova-Kostova, A.T.; Kasai, T.; Kamei, T.; et al. Molecular Basis for the Disruption of Keap1–Nrf2 Interaction via Hinge & Latch Mechanism. Commun. Biol. 2021, 4, 576.
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478.
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20.
- Olagnier, D.; Farahani, E.; Thyrsted, J.; Blay-Cadanet, J.; Herengt, A.; Idorn, M.; Hait, A.; Hernaez, B.; Knudsen, A.; Iversen, M.B.; et al. SARS-CoV2-Mediated Suppression of NRF2-Signaling Reveals Potent Antiviral and Anti-Inflammatory Activity of 4-Octyl-Itaconate and Dimethyl Fumarate. Nat. Commun. 2020, 11, 4938.
- Klotz, L.-O.; Sánchez-Ramos, C.; Prieto-Arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox Regulation of FoxO Transcription Factors. Redox Biol. 2015, 6, 51–72.
- Borniquel, S.; García-Quintáns, N.; Valle, I.; Olmos, Y.; Wild, B.; Martínez-Granero, F.; Soria, E.; Lamas, S.; Monsalve, M. Inactivation of Foxo3a and Subsequent Downregulation of PGC-1α Mediate Nitric Oxide-Induced Endothelial Cell Migration. Mol. Cell. Biol. 2010, 30, 4035–4044.
- Li, X.; Monks, B.; Ge, Q.; Birnbaum, M.J. Akt/PKB Regulates Hepatic Metabolism by Directly Inhibiting PGC-1α Transcription Coactivator. Nature 2007, 447, 1012–1016.
- Andrzejewski, K.; Jampolska, M.; Zaremba, M.; Joniec-Maciejak, I.; Boguszewski, P.M.; Kaczyńska, K. Respiratory Pattern and Phrenic and Hypoglossal Nerve Activity during Normoxia and Hypoxia in 6-OHDA-Induced Bilateral Model of Parkinson’s Disease. J. Physiol. Sci. 2020, 70, 16
- Wu, M.; Bian, Q.; Liu, Y.; Fernandes, A.; Taylor, A.; Pereira, P.; Shang, F. Sustained Oxidative Stress Inhibits NF-ΚB Activation Partially via Inactivating the Proteasome. Free Radic. Biol. Med. 2009, 46, 62–69
- Andrzejewski, K.; Jampolska, M.; Zaremba, M.; Joniec-Maciejak, I.; Boguszewski, P.M.; Kaczyńska, K. Respiratory Pattern and Phrenic and Hypoglossal Nerve Activity during Normoxia and Hypoxia in 6-OHDA-Induced Bilateral Model of Parkinson’s Disease. J. Physiol. Sci. 2020, 70, 16. [Google Scholar]
- Deumens, R.; Blokland, A.; Prickaerts, J. Modeling Parkinson’s Disease in Rats: An Evaluation of 6-OHDA Lesions of the Nigrostriatal Pathway. Exp. Neurol. 2002, 175, 303–317.
- Glinka, Y.Y.; Youdim, M.B.H. Inhibition of Mitochondrial Complexes I and IV by 6-Hydroxydopamine. Eur. J. Pharmacol. Environ. Toxicol. Pharmacol. 1995, 292, 329–332
- Tanaka, K.; Ogawa, N.; Asanuma, M. Molecular Basis of 6-Hydroxydopamine-Induced Caspase Activations Due to Increases in Oxidative Stress in the Mouse Striatum. Neurosci. Lett. 2006, 410, 85–89
- Tirmenstein, M.A.; Hu, C.X.; Scicchitano, M.S.; Narayanan, P.K.; McFarland, D.C.; Thomas, H.C.; Schwartz, L.W. Effects of 6-Hydroxydopamine on Mitochondrial Function and Glutathione Status in SH-SY5Y Human Neuroblastoma Cells. Toxicol. Vitr. 2005, 19, 471–479.
- Blum, D.; Torch, S.; Lambeng, N.; Nissou, M.-F.; Benabid, A.-L.; Sadoul, R.; Verna, J.-M. Molecular Pathways Involved in the Neurotoxicity of 6-OHDA, Dopamine and MPTP: Contribution to the Apoptotic Theory in Parkinson’s Disease. Prog. Neurobiol. 2001, 65, 135–172.
- Gong, P.; Deng, F.; Zhang, W.; Ji, J.; Liu, J.; Sun, Y.; Hu, J. Tectorigenin Attenuates the MPP+-induced SH-SY5Y Cell Damage, Indicating a Potential Beneficial Role in Parkinson’s Disease by Oxidative Stress Inhibition. Exp. Ther. Med. 2017, 14, 4431–4437.
- Vestuto, V.; Amodio, G.; Pepe, G.; Basilicata, M.G.; Belvedere, R.; Napolitano, E.; Guarnieri, D.; Pagliara, V.; Paladino, S.; Rodriquez, M.; et al. Cocoa Extract Provides Protection against 6-OHDA Toxicity in SH-SY5Y Dopaminergic Neurons by Targeting PERK. Biomedicines 2022, 10, 2009
- Chansiw, N.; Kulprachakarn, K.; Paradee, N.; Prommaban, A.; Srichairatanakool, S. Protection of Iron-Induced Oxidative Damage in Neuroblastoma (SH-SY5Y) Cells by Combination of 1-(N-Acetyl-6-Aminohexyl)-3-Hydroxy-2-Methylpyridin-4-One and Green Tea Extract. Bioinorg. Chem. Appl. 2021, 2021, 5539666
- Chen, D.; Kanthasamy, A.G.; Reddy, M.B. EGCG Protects against 6-OHDA-Induced Neurotoxicity in a Cell Culture Model. Park. Dis. 2015, 2015, 843906
- Semenza, G.L. HIF-1: Mediator of Physiological and Pathophysiological Responses to Hypoxia. J. Appl. Physiol. 2000, 88, 1474–1480.
- Chazelas, P.; Steichen, C.; Favreau, F.; Trouillas, P.; Hannaert, P.; Thuillier, R.; Giraud, S.; Hauet, T.; Guillard, J. Oxidative Stress Evaluation in Ischemia Reperfusion Models: Characteristics, Limits and Perspectives. IJMS 2021, 22, 2366.
- Irani, K. Oxidant Signaling in Vascular Cell Growth, Death, and Survival: A Review of the Roles of Reactive Oxygen Species in Smooth Muscle and Endothelial Cell Mitogenic and Apoptotic Signaling. Circ. Res. 2000, 87, 179–183.
- Saikumar, P.; Dong, Z.; Weinberg, J.M.; Venkatachalam, M.A. Mechanisms of Cell Death in Hypoxia/Reoxygenation Injury. Oncogene 1998, 17, 3341–3349
- Klein, C.; Westenberger, A. Genetics of Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a008888
- Klein, C.; Westenberger, A. Genetics of Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a008888.
- Martin, I.; Dawson, V.L.; Dawson, T.M. Recent Advances in the Genetics of Parkinson’s Disease. Annu. Rev. Genom. Hum. Genet. 2011, 12, 301–325.
- Scott, L.; Dawson, V.L.; Dawson, T.M. Trumping Neurodegeneration: Targeting Common Pathways Regulated by Autosomal Recessive Parkinson’s Disease Genes. Exp. Neurol. 2017, 298, 191–201
- Ittner, L.M.; Halliday, G.M.; Kril, J.J.; Götz, J.; Hodges, J.R.; Kiernan, M.C. FTD and ALS—Translating Mouse Studies into Clinical Trials. Nat. Rev. Neurol. 2015, 11, 360–366
- Haass, C.; Strooper, B.D. The Presenilins in Alzheimer’s Disease--Proteolysis Holds the Key. Science 1999, 286, 916–919.
- Dawson, T.M.; Golde, T.E.; Lagier-Tourenne, C. Animal Models of Neurodegenerative Diseases. Nat. Neurosci. 2018, 21, 1370–1379.
- Johnson, T.E.; de Castro, E.; Hegi de Castro, S.; Cypser, J.; Henderson, S.; Tedesco, P. Relationship between Increased Longevity and Stress Resistance as Assessed through Gerontogene Mutations in Caenorhabditis Elegans. Exp. Gerontol. 2001, 36, 1609–1617.
- Melov, S. Animal Models of Oxidative Stress, Aging, and Therapeutic Antioxidant Interventions. Int. J. Biochem. Cell Biol. 2002, 34, 1395–1400.
- Larsen, P.L. Aging and Resistance to Oxidative Damage in Caenorhabditis Elegans. Proc. Natl. Acad. Sci. USA 1993, 90, 8905–8909.
- Johnson, T.E. Increased Life-Span of Age-1 Mutants in Caenorhabditis Elegans and Lower Gompertz Rate of Aging. Science 1990, 249, 908–912
- Bradley, P.R. (Ed.) British Herbal Compendium: A Handbook of Scientific Information on Widely Used Plant Drugs; British Herbal Medicine Association: Bournemouth, UK, 1992.
- Bradley, P.R. (Ed.) British Herbal Compendium: A Handbook of Scientific Information on Widely Used Plant Drugs; British Herbal Medicine Association: Bournemouth, UK, 1992
- Yang, Y.; Liu, P.; Chen, L.; Liu, Z.; Zhang, H.; Wang, J.; Sun, X.; Zhong, W.; Wang, N.; Tian, K.; et al. Therapeutic Effect of Ginkgo Biloba Polysaccharide in Rats with Focal Cerebral Ischemia/Reperfusion (I/R) Injury. Carbohydr. Polym. 2013, 98, 1383–1388
- Achete de Souza, G.; de Marqui, S.V.; Matias, J.N.; Guiguer, E.L.; Barbalho, S.M. Effects of Ginkgo Biloba on Diseases Related to Oxidative Stress. Planta Med. 2020, 86, 376–386.
- Trebatická, J.; Ďuračková, Z. Psychiatric Disorders and Polyphenols: Can They Be Helpful in Therapy? Oxidative Med. Cell. Longev. 2015, 2015, 248529.
- Malik, J.; Choudhary, S.; Kumar, P. Plants and Phytochemicals for Huntington′s Disease. Pharmacogn. Rev. 2013, 7, 81.
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent Progress Regarding Kaempferol for the Treatment of Various Diseases (Review). Exp. Ther. Med. 2019, 18, 2759–2776.
- Zhou, M.; Ren, H.; Han, J.; Wang, W.; Zheng, Q.; Wang, D. Protective Effects of Kaempferol against Myocardial Ischemia/Reperfusion Injury in Isolated Rat Heart via Antioxidant Activity and Inhibition of Glycogen Synthase Kinase-3 β. Oxidative Med. Cell. Longev. 2015, 2015, 481405.
- Yang, C.-C.; Hsiao, L.-D.; Wang, C.-Y.; Lin, W.-N.; Shih, Y.-F.; Chen, Y.-W.; Cho, R.-L.; Tseng, H.-C.; Yang, C.-M. HO-1 Upregulation by Kaempferol via ROS-Dependent Nrf2-ARE Cascade Attenuates Lipopolysaccharide-Mediated Intercellular Cell Adhesion Molecule-1 Expression in Human Pulmonary Alveolar Epithelial Cells. Antioxidants 2022, 11, 782.
- Ma, T.; Lv, L.; Yu, Y.; Jia, L.; Song, X.; Xu, X.; Li, T.; Sheng, X.; Wang, H.; Zhang, J.; et al. Bilobalide Exerts Anti-Inflammatory Effects on Chondrocytes Through the AMPK/SIRT1/MTOR Pathway to Attenuate ACLT-Induced Post-Traumatic Osteoarthritis in Rats. Front. Pharmacol. 2022, 13, 783506.
- Zhou, L.J.; Zhu, X.Z. Reactive Oxygen Species-Induced Apoptosis in PC12 Cells and Protective Effect of Bilobalide. J. Pharmacol. Exp. Ther. 2000, 293, 982–988.
- Wang, F.; Huang, J.; Li, J.; Chen, K.; Zhang, X.; Zhang, Y.; Zhu, Y. Bilobalide, a Bioactive Compound on Sepsis-Induced Acute Lung Injury through Its Anti-Inflammatory and Antioxidative Activity. Pharmacogn. Mag. 2021, 17, 163.
- Tchantchou, F.; Lacor, P.N.; Cao, Z.; Lao, L.; Hou, Y.; Cui, C.; Klein, W.L.; Luo, Y. Stimulation of Neurogenesis and Synaptogenesis by Bilobalide and Quercetin via Common Final Pathway in Hippocampal Neurons. JAD 2009, 18, 787–798.
- Zhou, J.-M.; Gu, S.-S.; Mei, W.H.; Zhou, J.; Wang, Z.Z.; Xiao, W. Ginkgolides and Bilobalide Protect BV2 Microglia Cells against OGD/Reoxygenation Injury by Inhibiting TLR2/4 Signaling Pathways. Cell Stress Chaperones 2016, 21, 1037–1053.
- Jiang, H.; Qu, P. Effects of Ginkgo Biloba Leaf Extract on Local Renin-Angiotensin System through TLR4/NF-ΚB Pathway in Cardiac Myocyte. Exp. Ther. Med. 2017, 14, 5857–5862.
- He, G.; Yuan, C.; Hao, L.; Xu, Y.; Zhang, Z. GBE50 Attenuates Inflammatory Response by Inhibiting the P38 MAPK and NF-κB Pathways in LPS-Stimulated Microglial Cells. Evid. Based Complement. Altern. Med. 2014, 2014, 368598.
- Liu, F.-Q.; Gao, Q.; Wang, D.-D.; Zhang, Z.-X. Effects of GBE50 on LPS/ATP induced NLRP3 inflammasome activation in primary rat microglia. China J. Chin. Mater. Med. 2018, 43, 3346–3352
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301
- Chen, Z.; Cui, Q.; Cooper, L.; Zhang, P.; Lee, H.; Chen, Z.; Wang, Y.; Liu, X.; Rong, L.; Du, R. Ginkgolic Acid and Anacardic Acid Are Specific Covalent Inhibitors of SARS-CoV-2 Cysteine Proteases. Cell Biosci. 2021, 11, 45
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Di Pierro, F. A Role for Quercetin in Coronavirus Disease 2019 (COVID-19). Phytother. Res. 2021, 35, 1230–1236.
- Liu, Q.; Jin, Z.; Xu, Z.; Yang, H.; Li, L.; Li, G.; Li, F.; Gu, S.; Zong, S.; Zhou, J.; et al. Antioxidant Effects of Ginkgolides and Bilobalide against Cerebral Ischemia Injury by Activating the Akt/Nrf2 Pathway in Vitro and in Vivo. Cell Stress Chaperones 2019, 24, 441–452
- Smith, J.V.; Burdick, A.J.; Golik, P.; Khan, I.; Wallace, D.; Luo, Y. Anti-Apoptotic Properties of Ginkgo Biloba Extract EGb 761 in Differentiated PC12 Cells. Cell. Mol. Biol. 2002, 48, 699–707.
- Perianayagam, M.C.; Madias, N.E.; Pereira, B.J.G.; Jaber, B.L. CREB Transcription Factor Modulates Bcl2 Transcription in Response to C5a in HL-60-Derived Neutrophils. Eur. J. Clin. Investig. 2006, 36, 353–361
- Tchantchou, F.; Xu, Y.; Wu, Y.; Christen, Y.; Luo, Y. EGb 761 Enhances Adult Hippocampal Neurogenesis and Phosphorylation of CREB in Transgenic Mouse Model of Alzheimer’s Disease. FASEB J. 2007, 21, 2400–2408
- Hao, F.; Li, A.; Yu, H.; Liu, M.; Wang, Y.; Liu, J.; Liang, Z. Enhanced Neuroprotective Effects of Combination Therapy with Bone Marrow-Derived Mesenchymal Stem Cells and Ginkgo Biloba Extract (EGb761) in a Rat Model of Experimental Autoimmune Encephalomyelitis. Neuroimmunomodulation 2016, 23, 41–57
- Du, X.; Wang, X.; Geng, M. Alzheimer’s Disease Hypothesis and Related Therapies. Transl. Neurodegener. 2018, 7, 2.
- Andersson, U. The Cholinergic Anti-Inflammatory Pathway Alleviates Acute Lung Injury. Mol. Med. 2020, 26, 64.
- Ivic, L.; Sands, T.T.J.; Fishkin, N.; Nakanishi, K.; Kriegstein, A.R.; Strømgaard, K. Terpene Trilactones from Ginkgo Biloba Are Antagonists of Cortical Glycine and GABAA Receptors. J. Biol. Chem. 2003, 278, 49279–49285.
- Alexandris, N.; Lagoumintzis, G.; Chasapis, C.T.; Leonidas, D.D.; Papadopoulos, G.E.; Tzartos, S.J.; Tsatsakis, A.; Eliopoulos, E.; Poulas, K.; Farsalinos, K. Nicotinic Cholinergic System and COVID-19: In Silico Evaluation of Nicotinic Acetylcholine Receptor Agonists as Potential Therapeutic Interventions. Toxicol. Rep. 2021, 8, 73–83.
- Spiegel, R.; Kalla, R.; Mantokoudis, G.; Maire, R.; Mueller, H.; Hoerr, R.; Ihl, R. Ginkgo Biloba Extract EGb 761® Alleviates Neurosensory Symptoms in Patients with Dementia: A Meta-Analysis of Treatment Effects on Tinnitus and Dizziness in Randomized, Placebo-Controlled Trials. CIA 2018, 13, 1121–1127.
- Di Meo, F.; Cuciniello, R.; Margarucci, S.; Bergamo, P.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Ginkgo Biloba Prevents Oxidative Stress-Induced Apoptosis Blocking P53 Activation in Neuroblastoma Cells. Antioxidants 2020, 9, 279
- Wang, L.P.; Zhang, X.Y.; Liu, N.; Ma, Z.Z.; Fang, D.S. Comparison of Integrated Traditional Chinese and Western Medicine Therapy on Vascular Cognitive Impairment with No Dementia. Genet. Mol. Res. 2015, 14, 4896–4902.
- Gschwind, Y.J.; Bridenbaugh, S.A.; Reinhard, S.; Granacher, U.; Monsch, A.U.; Kressig, R.W. Ginkgo Biloba Special Extract LI 1370 Improves Dual-Task Walking in Patients with MCI: A Randomised, Double-Blind, Placebo-Controlled Exploratory Study. Aging Clin. Exp. Res. 2017, 29, 609–619.
- Kuo, L.-C.; Song, Y.-Q.; Yao, C.-A.; Cheng, I.H.; Chien, C.-T.; Lee, G.-C.; Yang, W.-C.; Lin, Y. Ginkgolide A Prevents the Amyloid-β-Induced Depolarization of Cortical Neurons. J. Agric. Food Chem. 2019, 67, 81–89
- Karavelioglu, Z.; Cakir-Koc, R. Preparation of Chitosan Nanoparticles as Ginkgo Biloba Extract Carrier: In Vitro Neuroprotective Effect on Oxidative Stress-Induced Human Neuroblastoma Cells (SH-SY5Y). Int. J. Biol. Macromol. 2021, 192, 675–683
- Wang, L.; Zhao, X.; Yang, F.; Wu, W.; Liu, Y.; Wang, L.; Wang, L.; Wang, Z. Enhanced Bioaccessibility in Vitro and Bioavailability of Ginkgo Biloba Extract Nanoparticles Prepared by Liquid Anti-solvent Precipitation. Int. J. Food Sci. Technol
- Zhao, Y.; Xiong, S.; Liu, P.; Liu, W.; Wang, Q.; Liu, Y.; Tan, H.; Chen, X.; Shi, X.; Wang, Q.; et al. Polymeric Nanoparticles-Based Brain Delivery with Improved Therapeutic Efficacy of Ginkgolide B in Parkinson’s Disease. IJN 2020, 15, 10453–10467
- Dhawan, S.; Kapil, R.; Singh, B. Formulation Development and Systematic Optimization of Solid Lipid Nanoparticles of Quercetin for Improved Brain Delivery. J. Pharm. Pharmacol. 2011, 63, 342–351
- Haghighi, P.; Ghaffari, S.; Arbabi Bidgoli, S.; Qomi, M.; Haghighat, S. Preparation, Characterization and Evaluation of Ginkgo Biloba Solid Lipid Nanoparticles. Nanomed. Res. J. 2018, 3, 71–78
- Xu, Z.; Feng, Q.; Wang, M.; Zhao, H.; Lin, Y.; Zhou, S. Green Biosynthesized Silver Nanoparticles With Aqueous Extracts of Ginkgo Biloba Induce Apoptosis via Mitochondrial Pathway in Cervical Cancer Cells. Front. Oncol.
- Wang, Q.; Ma, R.; Liu, P.; Cheng, G.; Yang, Q.; Chen, X.; Wu, Z.; Yuan, D.; Chen, T. Efficient Sustained-Release Nanoparticle Delivery System Protects Nigral Neurons in a Toxin Model of Parkinson’s Disease. Pharmaceutics 2022, 14, 1731
- Xu, Z.; Feng, Q.; Wang, M.; Zhao, H.; Lin, Y.; Zhou, S. Green Biosynthesized Silver Nanoparticles With Aqueous Extracts of Ginkgo Biloba Induce Apoptosis via Mitochondrial Pathway in Cervical Cancer Cells. Front. Oncol.
- Wang, Q.; Ma, R.; Liu, P.; Cheng, G.; Yang, Q.; Chen, X.; Wu, Z.; Yuan, D.; Chen, T. Efficient Sustained-Release Nanoparticle Delivery System Protects Nigral Neurons in a Toxin Model of Parkinson’s Disease. Pharmaceutics 2022, 14, 1731
