Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Transplantation
|
Infectious Diseases
Fungal infections are a significant source of morbidity in the lung transplant population via direct allograft damage and predisposing patients to the development of chronic lung allograft dysfunction. Prompt diagnosis and treatment are imperative to limit allograft damage. Aspergillus is among one of the most common sources of fungal infections in LTR.
- lung transplant
- fungal infections
- antifungals
1. Introduction
Fungal infections are common in lung transplant recipients (LTR). The cumulative 1-year incidence of fungal infections in LTR ranges from 10–22% [1]. In a population-based cohort study of about 9200 solid organ transplant (SOT) recipients, LTR had the highest incidence of invasive fungal infections (IFI) at 43 per 1000 person-years and a 10-year probability of 26.4% [2]. In the setting of prophylaxis, the prevalence of IFI within 180 days of lung transplant is 19.1 per 100 surgeries, with Aspergillus spp. accounting for 58% of non-Candida IFI [3]. The elevated incidence of fungal infections in LTR is of significant consequence since it has been directly linked to the development of chronic lung allograft dysfunction (CLAD), which has been associated with poor 3- and 5-year outcomes in LTR [4]. Furthermore, IFI in LTR is associated with the highest 1-year mortality out of all SOT recipients [2]. Indeed, the identification and treatment of fungal infections in LTR are crucial to limit the poor long-term outcomes associated with this common post-transplant complication.
LTR are more prone to fungal infections compared to other solid organ transplant recipients due to increased exposure to microorganisms via direct contact of the allograft with the environment [5]. LTR allografts will also have diminished elimination of microorganisms through impairment in mucociliary clearance and cough reflex, and respiratory tract structural abnormalities secondary to chronic respiratory disorders predispose LTR to microorganism colonization [5,6]. Receipt of immunosuppression and a reduction of blood flow to the site of infection through airway ischemia also reduce the host immune system’s ability to defend against infections [5,7]. Diagnosis of fungal infections following lung transplantation poses unique challenges as it can be hard to distinguish between colonization and active infection [6]. The multifactorial nature behind the increased risk of fungal infections in LTR results in the enlistment of various strategies to prevent and manage active fungal infections. Antifungal prophylaxis, either universal or pre-emptive, combined with routine post-transplant surveillance, has been implemented as a strategy to mitigate the risk of fungal infections [7,8]. Additionally, once infected, the degree of immunosuppression may be lowered in combination with the administration of antifungal agents. Indeed, the identification and treatment of fungal infections in LTR are crucial to limit the poor long-term outcomes associated with this common post-transplant complication.
2. Aspergillus
Aspergillus is among one of the most common sources of fungal infections in LTR. The incidence of invasive pulmonary aspergillosis in LTR has been reported as occurring in approximately 4–8% of LTR and the utilization of antimold prophylaxis has extended the average time of onset to 550 days post transplant [9,10]. Patients are at an increased risk for infections from Aspergillus if they are colonized within the first 12 months post-lung transplantation, have a single lung transplant, ≥3 episodes of supratherapeutic tacrolimus levels, if they experience anastomotic complications, airway/graft ischemia, reperfusion injury, bronchial anastomotic leaks, airway narrowing, CMV infection, or have had episodes of allograft rejection [11,12]. Tracheobronchial disease is the most common manifestation, and dissemination to other organs may occur late after transplant.
2.1. Diagnosis
A recent consensus document on IA in SOT recipients states that the approach to diagnosis should be multifaceted including histopathology, microbiology, serology, and imaging but that definitive diagnosis is hampered by a lack of prospective and high-quality studies in SOT [12].
(1,3)-β-D-glucan is a component of the fungal cell walls of Aspergillus, Candida, and Pneumocystis species. The Fungitell® assay is approved to detect (1,3)-β-D-glucan in the serum. A meta-analysis in mainly non-SOT immunocompromised patients demonstrated a sensitivity of 77% and specificity of 83% for IA [13]. In the lung transplant population, serum (1,3)-β-D-glucan was evaluated; there was no difference in median (1,3)-β-D-glucan values between those with and without IFI’s with a cutoff value of ≥60 pg/mL demonstrating a sensitivity of 64% with a specificity of 8%. False positives were linked to colonization of the respiratory tract and the receipt of renal replacement therapy within seven days of sample collection; false negatives were linked to the receipt of systemic antifungal therapy [14]. Two studies have evaluated (1,3)-β-D-glucan assays of the bronchoalveolar lavage (BAL) fluid in transplant recipients; median (1,3)-β-D-glucan values were similar between colonized and actively infected individuals [15]. One study utilized a cutoff of 41 pg/mL and found a sensitivity of 80% with 53% specificity [15]; the other study used a cutoff of 60 pg/mL with an associated sensitivity of 79% and specificity of 40% [16].
Galactomannan is a cell wall component of Aspergillus spp. that is released through the growth of the organism. Platelia™ Aspergillus antigen immunoenzymatic assay (EIA) can be used on serum or BAL fluid with a positive result if the serum or BAL fluid has an optical density (OD) of ≥0.5 [17]. Platelia™ assays have been demonstrated to have lower accuracy in SOT patients when compared to hematological malignancies or post-hematopoietic cell transplantation [17]. Specifically in LTR, using a positive OD cutoff of 0.5, 25% of patients with IA had a positive galactomannan serum sample; sensitivity marginally improved to 30% when the cutoff was raised to an OD of 0.66. Notably, most false positives occurred within the first two weeks following lung transplantation and sensitivity was 0% for detection of Aspergillus tracheobronchitis [18]. Galactomannan assays on BAL fluid to diagnose IA has been assessed in meta-analyses that mainly included non-lung transplant immunocompromised patients, demonstrating that it could be successfully employed with a positive OD cutoff value of 1.0 showing higher sensitivity and lower specificity than serum galactomannan assays [19]. In LTR, three studies have utilized a positive OD cutoff value of ≥0.5 in BAL fluid for IA diagnosis, demonstrating a sensitivity of 60–100% and specificity of 89–100% [20,21,22]. Another evaluation of the Aspergillus galactomannan assay on BAL fluid in LTR identified an optimal OD cutoff of 1.5 which gave a sensitivity of 100% and specificity of 90%; higher BAL galactomannan OD indexes were observed in single lung transplants when compared to bilateral transplants. With respect to false galactomannan results, receipt of piperacillin/tazobactam has historically been linked to false positive galactomannan results; however, recent publications have failed to show a significant association between false positive galactomannan results and receipt of piperacillin/tazobactam when multiple lots from varying manufacturers were assessed [23,24,25]. Cross-reactivity resulting in positive Platelia™ Aspergillus galactomannan results has been reported to occur in 50% of patients with Paracoccidioides brasiliensis, 67% of patients with Histoplasma capsulatum, 63% of those with Cryptococcus neoformans, and 37% of patients with Cryptococcus gattii infections [26].
PCR testing on BAL fluid in immunocompromised patients has been evaluated in a meta-analysis for diagnosing proven or probable IA and had a sensitivity of 75% with 94% specificity in the setting of heterogenous PCR techniques [27]. Aspergillus PCR testing has historically been limited by the inability to discriminate between colonization of the respiratory tract and active infection, the inability to determine Aspergillus subspecies, and the lack of a standardized PCR methodology. One study in LTR evaluated the use of real-time Viracor pan-Aspergillus PCR of BAL samples and compared results to BAL galactomannan assays in 137 patients. The optimal quantification cycle (Cq) for Aspergillus was determined to be ≤35, which led to a sensitivity of 100% with a specificity of 88% and was significantly lower than patients who were colonized with Aspergillus, although 81% of false positive PCR results were determined to be due to airway colonization and incidences of false positive PCRs were significantly higher than false positive galactomannan assay results [20].
A lateral flow device (LFD) qualitatively detects an Aspergillus extracellular glycoprotein antigen via a monoclonal antibody. In immunocompromised patients, BAL fluid was evaluated using LFD, galactomannan assay, PCR, (1,3)-β-D-glucan assay, and mycology culture; LFD demonstrated a sensitivity of 80% with 95% specificity [28]. In LTR, the use of LFD on BAL fluid showed similar results with a sensitivity of 91% and specificity of 83% for the detection of invasive pulmonary Aspergillus (IPA) [29]. A more recent study of BAL fluid point-of-care LFD in a heterogeneous immunocompromised population, including those critically ill, demonstrated a lower sensitivity (58–69%) and specificity (68–75%) for the diagnosis of IPA [30].
CT imaging of Aspergillus pulmonary infections within LTR has been studied with high-resolution computed tomography, which demonstrated that abnormalities were bilateral in 87%; among those with unilateral abnormalities, all were single lung transplants with the transplanted organ being affected in two out of three cases. Centrilobar tree-in-bud nodules with bronchial wall thickening were present in 65% of cases, consolidation and ground-glass opacities in 22%, and large nodules with or without halo sign were present in 13% of patients [31].
2.2. Treatment
Per IDSA guidelines, voriconazole is the first-line agent for the management of IA [32]. In a randomized controlled trial of 277 immunocompromised patients (mainly non-SOT) with definite or probable IA that compared voriconazole vs. amphotericin B, survival in the voriconazole group was 70.8% vs. 57.9% in the amphotericin B group (HR, 0.59, 95% CI, 0.40–0.88) [33]. The voriconazole group experienced fewer adverse drug reactions compared to the amphotericin B group, but also had an increased rate of transient visual disturbances. Isavuconazole is a second-generation triazole antifungal that has less drug–drug interactions with CYP3A4 substrates, such as tacrolimus and cyclosporine compared to other triazole antifungals, making it a desirable option for IA treatment in SOT recipients. However, there is limited evidence for its use following lung transplant. In the SECURE trial, which compared isavuconazole to voriconazole for the management of invasive mold infections, isavuconazole was shown to be non-inferior to voriconazole while also having a reduced incidence of hepatobiliary disorders, visual disorders, and skin or subcutaneous tissue disorders [34]. Twenty percent of patients were immunocompromised, but there were no lung transplant recipients. Similarly, posaconazole has been identified as a therapeutic option for IA. A phase 3, randomized controlled trial identified that posaconazole was non-inferior to voriconazole in survival up to day 42, while also resulting in fewer treatment-emergent adverse events than voriconazole [35]. While there were no lung transplant recipients in the trial, there was a significant population of hematopoietic stem cell transplant recipients, and patients receiving treatment with T-cell immunosuppressants or prolonged courses of corticosteroids. Ultimately, while only voriconazole has been specifically studied in LTR, posaconazole or isavuconazole may be viable first-line alternatives given their more favorable safety profile, as well as a diminished effect of drug interactions with isavuconazole.
Alternative therapies may be considered in patients who are unable to tolerate voriconazole, posaconazole, or isavuconazole. Possible strategies include itraconazole; echinocandins such as caspofungin or micafungin; or lipid formulations of amphotericin B [32]. Table 1 contains information on antifungal interactions, adverse reactions, and recommended dosing for aspergillosis.
Table 1. Antifungal considerations in SOT.
| Drug | Uses | Immunosuppressant Drug Interactions | Key Adverse Drug Reactions | Dosing (Pulmonary Infections) |
|---|---|---|---|---|
| Fluconazole | Candida (non-glabrata); Cryptococcus; Coccidioides; Blastomyces (alternative). |
Tacrolimus: 50% increase in serum tacrolimus levels [36]. Sirolimus: 28–70% increase in serum sirolimus levels [37]. Everolimus: 2.8-fold decrease in everolimus clearance [38]. Cyclosporine: approximately 150% increase in serum cyclosporine levels [36]. |
QTc prolongation, hepatotoxicity [39] | Candida a [40]: 800 mg on day 1, followed by 400 mg daily; duration dictated by extent of dissemination and resolution of signs/symptoms. Cryptococcus b [41]: 400 mg daily for 6–12 months followed by chronic suppression. Coccidioides [42]: 400–1200 mg daily for 6–12 months followed by chronic suppression. Blastomyces b [43]: 400–800 mg daily for 6–12 months. |
| Itraconazole | Aspergillus (alternative); Coccidioides; Histoplasma; Blastomyces. |
Tacrolimus: significant increases in concentrations requiring a 50–75% dose reduction [44]. Sirolimus: significant increase in sirolimus concentrations anticipated [45]. Everolimus: 3.9-fold increase in everolimus Cmax and 15-fold increase in everolimus AUC [46]. Cyclosporine: 50–75% cyclosporine dose reductions have been required in LTRs [47,48]. |
Hepatotoxicity, peripheral neuropathy, hearing loss, CNS depression, QTc prolongation [45]. Boxed warning: heart failure exacerbation through negative inotropic effects [45]. |
Candida c [45] Aspergillusc,d [45]: 200–400 mg twice daily for 6–12 weeks. Coccidioides c [49]: 200 mg twice daily for ≥12 months followed by chronic suppression. Histoplasma c [42]: 200 mg twice daily for ≥12 months followed by chronic suppression. Blastomyces c [43]: 200 mg twice daily for 6–12 months. |
| Voriconazole | Aspergillus; C. glabrata (alternative); C. krusei (alternative); Cryptococcus (alternative); Coccidioides (alternative); Histoplasma (step-down, alternative) [50]. Blastomyces (alternative); Scedosporium (alternative); Fusarium [51]. |
Tacrolimus: 2-and 3-fold increases of tacrolimus Cmax and AUC, respectively [52]. Sirolimus: 4.5-to 11-fold increase in sirolimus AUC [52]. Everolimus: 8.2-fold increase in everolimus concentration/dose ratio; everolimus dose reductions of 67% have been needed [53,54]. Cyclosporine: 1.7-fold increase in cyclosporine AUC and 2.5-fold increase in cyclosporine minimum plasma concentration [52,55]. |
Acute kidney injury, QTc prolongation, hepatotoxicity, periosteal disease, and visual disturbances [52]. | Aspergillus e,f [12], Cryptococcus f [56]: IV: 6 mg/kg twice daily for 2 doses, then 4 mg/kg twice daily; oral: 200 mg twice daily for ≥6 weeks. Candida f [40]: 400 mg twice daily for 2 doses, then 200–300 mg twice daily; duration dictated by extent of dissemination and resolution of signs/symptoms. Coccidioides f [57], Histoplasma f [52]: 400 mg twice daily for 2 doses, then 200 mg twice daily for ≥12 months followed by chronic suppression. Blastomyces f [4]: 400 mg twice daily for 2 doses, then 200 mg twice daily for 6–12 months. Scedosporium f [51]: IV: 6 mg/kg twice daily for 2 doses, then 4 mg/kg twice daily; oral: 400 mg twice daily for 2 doses, then 200–300 mg twice daily for a prolonged duration. Fusarium f,g [51]: IV: 6 mg/kg twice daily for 2 doses, then 4 mg/kg twice daily followed by step-down to oral 200 mg twice daily once improvement on IV for a prolonged duration. |
| Posaconazole | Aspergillus Candida (alternative); Cryptococcus (alternative); Mucorales (alternative); Coccidioides (alternative); Histoplasma (step-down, alternative); Blastomyces (alternative); Fusarium (alternative) [58]. |
Tacrolimus: ~120% increase in tacrolimus Cmax and ~350% increase in tacrolimus AUC [59,60]. Sirolimus: 8.9-fold increase in sirolimus AUC [61,62]. Everolimus: 3.5-fold increase in everolimus Cmin/dose ratio [63]. Cyclosporine: reductions in cyclosporine dose of 14–29% have been required [59]. |
Hepatotoxicity, QTc prolongation [64]. | Aspergillus h [32]: tablets (preferred): 300 mg twice daily for 2 doses, then 300 mg daily; suspension: 200 mg three times daily or 400 mg twice daily for ≥6 months. Candida [65]: Tablet: 300 mg daily; suspension: 400 mg twice daily; duration dictated by extent of dissemination and resolution of signs/symptoms. Cryptococcus [57]: 300 mg twice daily for 2 doses, then 300 mg daily; suspension: 200 mg three times daily or 400 mg twice daily for 6–12 months followed by chronic suppression. Mucorales step-down [66], Fusarium: tablets/IV: 300 mg twice daily for 2 doses, then 300 mg daily (suspension not recommended) for a prolonged duration. Coccidioides [57], Histoplasma [67]: tablets: 300 mg twice daily for 2 doses, then 300 mg daily; suspension: 200 mg three times daily or 400 mg twice daily for ≥12 months followed by chronic suppression. Blastomyces [68]: tablets: 300 mg twice daily for 2 doses, then 300 mg daily; suspension: 200 mg three times daily or 400 mg twice daily for 6–12 months. |
| Isavuconazole | Aspergillus Candida (alternative); Cryptococcus (alternative); Mucorales (alternative); Coccidioides (alternative); Histoplasma (step-down, alternative); Blastomyces (alternative). |
Tacrolimus: dose/concentration ratio has been decreased by 30% [69]. Sirolimus: likely to significantly increase sirolimus levels [70]. Everolimus: likely to significantly increase everolimus levels [71]. Cyclosporine: AUC and Cmax have been increased by 29% and 6%, respectively [62,72]. |
QTc shortening, hepatotoxicity [70]. | Aspergillus [12]: 372 mg every 8 h for 6 doses, then 372 mg daily for ≥6 weeks. Candida [70]: 372 mg every 8 h for 6 doses, then 372 mg daily; duration dictated by extent of dissemination and resolution of signs/symptoms. Cryptococcus [73]: 372 mg every 8 h for 6 doses, then 372 mg daily for 6–12 months followed by chronic suppression. Mucorales: 372 mg every 8 h for 6 doses, then 372 mg daily for a prolonged duration [66]. Coccidioides [73], Histoplasma: 372 mg every 8 h for 6 doses, then 372 mg daily for ≥12 months followed by chronic suppression. Blastomyces [73]: 372 mg every 8 h for 6 doses, then 372 mg daily for 6–12 months. |
| Caspofungin | Aspergillus (alternative) [32]. Candida Mucorales (alternative in combination with amphotericin B) [66]. |
Tacrolimus decrease in Cmax by 16%, Cmin by 26%, and AUC by 20% [74]. | Hypotension, peripheral edema, tachycardia, phlebitis, and elevated liver enzymes [74]. | Aspergillus (part of combination therapy): 70 mg on first day, then 50 mg daily for ≥6 weeks. Candida [65]: 70 mg on first day, then 50 mg daily; duration dictated by extent of dissemination and resolution of signs/symptoms. Mucorales (part of combination therapy): 70 mg on first day, then 50 mg daily for a prolonged duration. |
| Anidulafungin | Aspergillus (alternative) [32]. Candida Mucorales (alternative in combination with amphotericin B) [66]. |
None. | Hypo/hypertension, hypokalemia, hypomagnesemia, and peripheral edema [75]. | Aspergillus (part of combination therapy): 200 mg on first day, then 100 mg daily for ≥6 weeks. Candida: 200 mg on first day, then 100 mg daily; duration dictated by extent of dissemination and resolution of signs/symptoms [65]. Mucorales (part of combination therapy): 200 mg on first day, then 100 mg daily for a prolonged duration. |
| Micafungin | Aspergillus (alternative) [32]. Candida Mucorales (alternative in combination with amphotericin B) [66]. |
Sirolimus AUC may increase by 21% [76]. Cyclosporine: 1.7-fold increase in cyclosporine serum concentrations [77,78]. |
Phlebitis [76]. | Aspergillus (part of combination therapy): 100–150 mg daily for ≥6 weeks. Candida [65]: 100 mg daily; duration dictated by extent of dissemination and resolution of signs/symptoms. Mucorales (part of combination therapy): 100–150 mg daily for a prolonged duration. |
| Amphotericin B deoxycholate | Aspergillus (alternative); Candida (alternative); Cryptococcus (alternative); Coccidioides (alternative) [43]. Blastomyces (alternative). |
None. | Dose-dependent nephrotoxicity, infusion reactions, transaminitis, hypokalemia, hypomagnesemia, and hypocalcemia [79]. | Aspergillus i [5]: 1–1.5 mg/kg/day for ≥6 weeks. Candida i [40]: 0.5–0.7 mg/kg/day; duration dictated by extent of dissemination and resolution of signs/symptoms. Cryptococcus i [56] (in combination with flucytosine or fluconazole): 0.7–1 mg/kg/day for ≥2 weeks followed by step-down therapy. Coccidioides i [42]: 0.5–1 mg/kg/day until clinical improvement followed by step-down therapy. Blastomyces i [43]: 0.7–1 mg/kg/day for 1–2 weeks followed by step-down therapy. |
| Liposomal amphotericin B | Aspergillus (alternative); Candida (alternative); Cryptococcus; Mucormycosis; Coccidioides [42]. Histoplasma [42]. Blastomyces. |
Dose-dependent nephrotoxicity (less common than with amphotericin deoxycholate), infusion reactions, transaminitis, hypokalemia, hypomagnesemia, and hypocalcemia [80]. | Aspergillus j [13]: 3–5 mg/kg/day for ≥6 weeks. Candida j [40]: 3–5 mg/kg/day; duration dictated by extent of dissemination and resolution of signs/symptoms. Cryptococcus j [41] (in combination with flucytosine or fluconazole): 3–4 mg/kg/day for ≥2 weeks followed by step-down therapy. Mucorales j [66]: 5–10 mg/kg/day for a prolonged duration. Coccidioides j [42]: 3–5 mg/kg/day until clinical improvement followed by step-down therapy. Histoplasma j [42]: 3–5 mg/kg/day for 1–2 weeks followed by step-down therapy. Blastomyces j [43]: 3–5 mg/kg/day for 1–2 weeks followed by step-down therapy. |
|
| Amphotericin B lipid complex | Aspergillus (alternative); Candida (alternative); Cryptococcus; Mucorales; Coccidioides [42]. Histoplasma [42]. Blastomyces. |
Aspergillus k [12]: 5 mg/kg/day for ≥6 weeks [13]. Candida k [40]: 3–5 mg/kg/day; duration dictated by extent of dissemination and resolution of signs/symptoms. Cryptococcus k [56] (in combination with flucytosine or fluconazole): 5 mg/kg/day for ≥2 weeks followed by step-down therapy [41]. Mucorales k [66]: 5–10 mg/kg/day for a prolonged duration. Coccidioides k [42]: 3–5 mg/kg/day until clinical improvement followed by step-down therapy. Histoplasma k [42]: 5 mg/kg/day for 1–2 weeks followed by step-down therapy. Blastomyces k [43]: 3–5 mg/kg/day for 1–2 weeks followed by step-down therapy. |
Abbreviations: AUC: area under the curve; LTRs: lung transplant recipients; Cmin: minimum blood plasma concentration; Cmax: maximum blood plasma concentration; CNS: central nervous system. Dose adjustments indicated for estimated glomerular filtrate rate < 50 mL/min. (a) Weight-based dosing should be considered in obesity; (b) initial treatment for mild disease, otherwise amphotericin B lipid complex is indicated prior to initiation; (c) solution preferred; (d) goal trough level after 4–7 days of therapy (combined hydroxyitraconazole and itraconazole) of 0.5–3 mcg/mL; (e) goal trough level after 4–7 days of therapy of 1–5.5 mcg/mL; (f) use adjusted body weight for calculations; (g) consider combination therapy in severe disease; (h) goal trough level after ≥7 days of therapy of ≥1 mg/L; (i) use adjusted body weight for calculations but actual body weight can be considered for severe infections, suggested maximum dose of 150 mg daily; (j) use actual body weight for calculations; recommended maximum dose of 600 mg; (k) use actual body weight for calculations; recommend maximum dose of 500 mg.
Bronchial anastomotic sites may undergo transient devascularization, and thus be more susceptible to ischemic injury. Thus, utilization of systemic agents may provide little benefit in patients with this complication in the setting of impaired blood flow to the ischemic site, and inhaled antifungal agents may be preferred. However, there is a low quality of evidence supporting the use of inhaled antifungals for IA [81]. Inhaled amphotericin B is often used as a prophylactic agent, but has demonstrated efficacy when used as an adjunct to systemic voriconazole, caspofungin, or amphotericin B for the treatment of IA [82]. Inhaled voriconazole has resulted in clinical improvement when utilized as monotherapy or adjunct to systemic caspofungin and liposomal amphotericin B [83,84]. It should be noted that the evidence is limited to case reports and case series for these agents, and further higher-quality studies are needed. A case vignette of a lung transplant recipient with aspergillosis is presented in Table 2 with the accompanying computed tomography image presented in Figure 1.
Table 2. Case vignettes of lung transplant recipients with Aspergillosis, Cryptococcosis, and Histoplasmosis.
| Patient | Presentation | Diagnosis | Treatment | Outcome |
|---|---|---|---|---|
| 1: Aspergillosis | 53 YO, 5 weeks post-transplant. Received antithymocyte globulin and carfilzomib 2 weeks prior. Symptom of desaturations. |
Chest CT: small right basilar empyema, partial collapse of left lower lobe, bilateral ground glass opacities, and septal thickening (Figure 1). Chest and pleural tissue culture from decortication procedure: A. fumigatus. |
Voriconazole 6 mg/kg for 2 doses followed by 4 mg/kg daily. Voriconazole changed to liposomal amphotericin B after one week. Five weeks later, daily intrapleural voriconazole irrigation added for one week. |
Systemic voriconazole stopped after one week for elevated hepatic function tests, intrapleural voriconazole stopped for bloody sputum. Aspergillus not redemonstrated in cultures. Patient death 4 months later due to bacterial sepsis. |
| 2: Cryptococcosis | 49 YO, 7 years post transplant. Received rituximab 3 months prior. Symptoms of headache, confusion, and photophobia. |
MRI: hydrocephalus. CT chest: multifocal nodular abnormalities. LP: opening pressure 36 cm, CSF 65% neutrophils, protein 107, glucose 14, RBC 24, WBC 17. India ink stain: yeast. Serum and CSF antigen titer: ≥1:2560. CSF culture: C. neoformans. |
Liposomal amphotericin 5 mg/kg + flucytosine for 16 days, then step-down to fluconazole 400 mg daily. Six days after step-down, liposomal amphotericin B restarted for altered mental status and cerebral swelling. |
Progressive renal dysfunction to 1.5× baseline serum creatinine at time of fluconazole step-down. Death 48 h after cerebral swelling noted on imaging. |
| 3: Histoplasmosis | 38 YO, 14 years post transplant. Symptoms of low-grade fever and weight loss prompted colonoscopy. |
CT chest/abdomen/pelvis: bowel wall thickening, lymphadenopathy, no acute pulmonary changes. Colonoscopy biopsy: Histoplasma (Figure 2). Histoplasma blood antibody: negative. Histoplasma urine antigen: 7.01 ng/mL. |
Liposomal amphotericin 5 mg/kg for 7 days, then step-down to itraconazole 200 mg TID for 9 doses, followed by 200 mg BID Itraconazole trough 1.5 mcg/mL. |
Remains on itraconazole after two years without significant side effects. Colonic thickening resolved five months after treatment initiation. Histoplasma urine antigen decreased to 0.77 ng/mL after 8 weeks of treatment. |
Abbreviations: CSF: cerebrospinal fluid; CT: computed tomography; LP: lumbar puncture; MRI: magnetic resonance imaging; RBC: red blood cell count; TID: three times daily; WBC: white blood cell count; YO: years old.
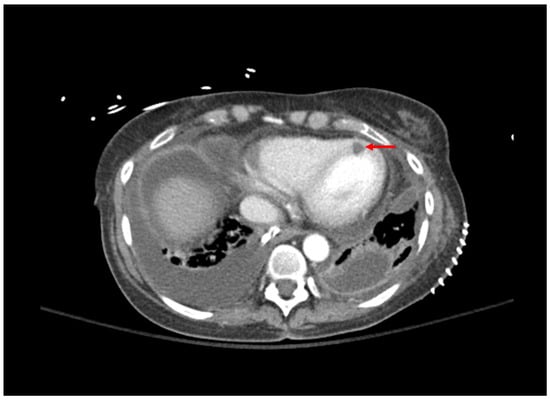
Figure 1. Computed tomography of the chest; bilateral pleural effusions grew Aspergillus fumigatus. Area of myocardial hypoperfusion consistent with fungal myocarditis is demonstrated by red arrow.
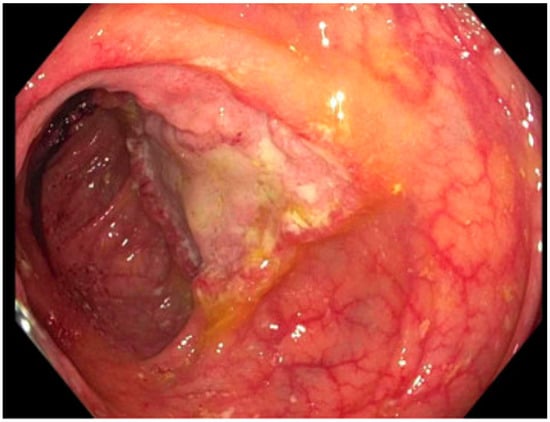
Figure 2. Ulcerated mass in the ascending colon; cold forceps biopsy yielded Histoplasma capsulatum.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens12050694
This entry is offline, you can click here to edit this entry!
