Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Composites
Lyotropic liquid crystals (LLCs) are promising templates for active layer materials due to their inherently uniform and controllable pore size, ranging from 0.2 to 5 nm. Membranes formed by LLC template materials possess low surface roughness and high hydrophilicity, which result in higher membrane-fouling resistance. Moreover, mesophases such as hexagonal and lamellar are reorientable, enabling water channels to align perpendicularly to the membrane surface and increasing water permeance.
- lyotropic liquid crystal
- nanofiltration
- interfacial structure
1. Introduction
The reduction in the supply of freshwater has prompted governments and scientists to develop water purification technologies [1]. As seawater makes up 96.5% of the total global water supply, seawater desalination is the most promising way to increase potable water supplies [2]. However, desalination consumes 6–7 times more energy than conventional water treatment methods to purify an equivalent amount of water [3]. In a common desalination plant, the membrane unit accounts for 85% of the overall energy consumption [4]. Therefore, improving membrane efficiency is crucial in reducing overall desalination energy consumption and further popularizing desalination techniques.
Thin-film composite (TFC) membranes, consisting of an active separation layer, porous support, and polyester non-woven backing layer, are considered a state-of-the-art design for seawater desalination membranes [5,6,7]. The active layer of commercial TFC membranes is typically a nonporous aromatic cross-linked polyamide (PA) [8]. Although this type of TFC is widely used, it is limited by its low permeability (<1 Lm−2 h−1 bar−1 μm) [9,10], non-uniform pore size [11], and low fouling resistance [7]. Lyotropic liquid crystals (LLCs) are promising templates for active layer materials due to their inherently uniform and controllable pore size, ranging from 0.2 to 5 nm [9,12,13,14,15,16]. Membranes formed by LLC template materials possess low surface roughness and high hydrophilicity, which result in higher membrane-fouling resistance [17]. Moreover, mesophases such as hexagonal and lamellar are reorientable, enabling water channels to align perpendicularly to the membrane surface and increasing water permeance.
Current studies on fabricating TFC membranes based on the LLC template active layer all directly form the LLC mesophase on substrates since this method guarantees interface [10]. However, research has shown that the surface properties of the substrate, including hydrophilicity and heterogeneity, could significantly affect the types and shapes of surfactant mesophases [18]. As LLCs are also formed by surfactants, it is reasonable to wonder about the accurate structures at the LLC/substrate interface. However, this essential part has been overlooked.
Studies on the conventional PA-based active layer show that the middle porous substrate of TFC not only regulates the water transport path but also interferes with the structure formation of the active layer [19]. For example, the funnel effect leads to a longer transport pathway of water on the substrate with low porosity [20,21]. Regarding structure formation interferences, the nanovoids formation on the PA surface has been effectively controlled by the surface pore size of the substrates, which can be explained by a volcano-like model [22] and the confinement effect [23,24]. In addition, the infusion depth of the PA layer in the substrate was found to be altered by both surface pore size and hydrophilicity [25,26,27]. Commercial polymer substrates are usually limited by their material nature, which is hydrophobic, has less porosity, and cannot enable the top layer to exert functions effectively.
Fortunately, the limitations of polymer substrates can be remedied by emerging surface modification and by introducing an interlayer between the substrate and top layer [28,29]. Surface modification methods, including surface deposition and surface grafting, usually focus on tackling the polymer surface hydrophobicity [29], while introducing an interlayer could bring additional benefits, such as adjusting the overall water channel [30], restricting the active layer infusion, and facilitating the formation of a uniform and defect-free top layer [31].
In addition to optimizing the surface properties of the substrates, accurately detecting the LLC/substrate interfacial structures is also of great importance for optimizing the interface. Microscopic methods such as scanning electron microscopy and transmission electron microscopy are usually destructive to the membranes and limited to probing small sample areas. X-ray and neutron techniques are more promising in non-destructively observing the buried LLC/substrate interfacial structures. The grazing incidence mode scattering technique is the most direct tool to identify the LLC phase structure at the interface by choosing a suitable beam incident angle [32], while neutron enables localization of the monomer molecules at the interface by contrast matching the deuterated amphiphile molecules and the mixtures of deuterated and hydrogen solvents [33].
2. Fabrication of LLC Active Layer with Suitable Structure Retention
The structure of TFC membranes based on the LLC template active layer typically consists of a top LLC template layer and a porous substrate. The porous substrates are typically commercially purchased microfiltration or ultrafiltration membranes or self-prepared (phase inverse method) porous membranes. This section presents the main efforts made in the fabrication of the LLC active layer on porous membranes, focusing on LLC phase behavior, structure retention, and a comparison of the resulting membranes structures and performances.
2.1. LLC Precursor on Porous Substrates
2.1.1. LLC Phases
LLCs are assemblies composed of amphiphilic molecules that contain a hydrophilic headgroup and a hydrophobic organic tail in the presence of a polar solvent, usually water. The amphiphilic nature of these molecules drives them to phase separation upon the addition of polar solvent, resulting in the formation of ordered hydrophobic and hydrophilic domains with water–oil interfaces determined by hydrophilic headgroups. The most common phases of LLC are lamellar (L), hexagonal (H), bi-continuous cubic (Q), and discontinuous cubic (I). Among these, the normal hexagonal phase (HI), inverted hexagonal phase (HII), and normal bi-continuous cubic (QI) are most commonly used as the template for fabricating top layers [10,16,34,35], as shown in Figure 1. Saadat and his coworkers have summarized the reactive and non-reactive surfactants that have been used to form these mesophases [17]. Compared to other mesophases, the hexagonal mesophase template materials are more promising for fabricating top layers with pores parallel to the macroscopic transport direction, i.e., vertical to the membrane surface. Such a tubular HLLC domain was verified to be reoriented using several up-to-date techniques, including magnetic field [14,36,37,38,39], electrical fields [40,41,42], shear force [10,12,43,44,45,46], and confinement methods [13,47].
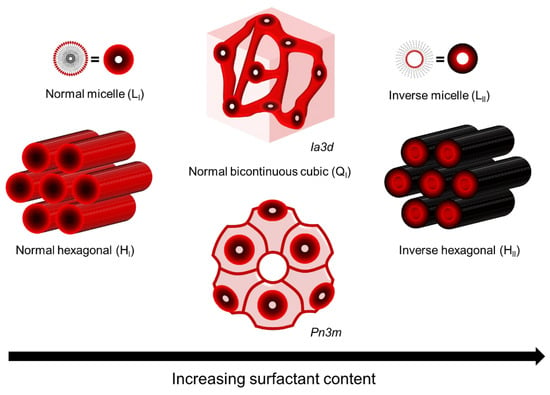
Figure 1. LLC phases used as template for fabricating top layer.
2.1.2. LLC Phase Behavior
A lipid monolayer possessing a spontaneous curvature can be bent with a concave surface to form cylinders, which is the basis of the LLC phase transition. The free energy of bending a thin surfactant layer can be calculated by [48]:
where E is the energy per lipid area, C1 and C2 are the principal curvatures, kc and kg are constants called the rigidity and Gaussian curvature constants, and C0 is referred to as the spontaneous curvature. C1, C2, and C0 are zero for the lamellar phase, and C1 + C2 = 0 for the bi-continuous cubie phase, while in the hexagonal phase, one of the principle curvatures is zero [2,48]. Therefore, for the cylinder phase, the free energy of bending a thin surfactant layer can be transferred to [48]:
where lower free energy of bending a thin layer corresponds to a higher spontaneous C0. However, this theory cannot explain the energy changes during the phase transition between the lamellar and hexagonal phases since the C0 is zero for the lamellar phase. In this case, the hydrocarbon packing energy theory was introduced [49,50,51]. The lamellar phase possesses a low hydrocarbon packing energy and a high bending energy, which means that the lower hydrocarbon packing energy drives the phase transition from the lamellar to the hexagonal phase when increasing the C0 of the flat layer. Therefore, the competition between the curvature on the interface of the mesophases and hydrocarbon packing mainly determines the structure of the LLC phase [48]. The temperature [52], pressure [53,54], pH value [55], and introducing agents such as inorganic salts [56,57] and alkanes [58] have been found to modulate the packing and bending energies of the LLC system. For instance, the decrease in temperature was found to increase the radius of the water core of the HII, which increases the hydrocarbon packing energy and forms a cylinder with a lower spontaneous curvature of the system. In this case, the introduction of a long-chain alkane can decrease the packing energy and increase the spontaneous curvature of the system, which stabilizes the LLC phase [48]. Efforts to control the LLC phase structure, LLC unit cell size, and diameter of the water phase in LLC have been motivated by many applications, including drug release and protein loading [59,60,61].
2.1.3. Polar Solvent
Water is the most typical polar solvent used in LLC mesophase. However, the inevitable evaporation of water limits the retention of LLC phase structures. This problem can be addressed by replacing the water with other polar organic solvents with lower volatility, such as glycerol, ionic liquids, ethylene glycol, N-methylsydone, formamide and its derivatives, and propylene carbonate [62,63,64]. These solvents have been studied to form LLC phases with alternative surfactants.
2.2. Ultrathin LLC Film Formation on Porous Substrates
2.2.1. LLC Templating
LLC mesophases suffer from poor mechanical and thermal properties, which limit their applications. Therefore, they are often used as templates to be polymerized into polyLLCs to overcome these limitations. Two common routes can be implemented to achieve robust polyLLCs: synergistic and transcriptive templating. The synergistic method is more direct since it cures the polymerizable surfactant directly, while the latter one cures the introduced monomers or crosslinkers in the confined area in the LLC template. Generally, self-assembly is a process by which molecules move to minimize their free energy. When transferring the LLC parent template to the polymer, the entropy of the system decreases, while the enthalpy of the isolated system commonly undergoes almost no change during the polymerization. Therefore, an increase in the free energy of the system will inevitably occur, leading to the collapse of the template structure. Two strategies can be used to avoid the loss of the parent structure [65]:
-
Establishing strong enough thermodynamic interactions between the surfactant template and polymer;
-
Increasing the system viscosity and chain entanglement by forming covalent and limiting species diffusion.
The fast formation of covalent bonds between monomers and the increase in the viscosity of the system by polymerization are reported to be the best strategies to retain the LLC structure, which restricts the diffusivity of the objects in the system constantly. Compared to synergistic templating, retaining the structure during the curing is more challenging for transcriptive templating since there are more movable objects in the transcriptive systems. Even so, a suitable choice of chemistry structure for monomers and initiators and the introduction of crosslinkers, polymerizable surfactant [66], silica network [67], and block copolymer [68] can increase the possibility of structure retention.
2.2.2. Fabrication Process
Roll-casting, hot or cold pressing, and spin-coating were used to fabricate LLC-based TFC membranes, as shown in Figure 2A. To fabricate a robust LLC-based TFC membrane, the inverse hexagonal (HII) phase was first roll-cast onto the commercial microporous polysulfone (Psf) support. Although this fabrication method is facile, the inevitable evaporation of water causes limited retention of HII phase structures.
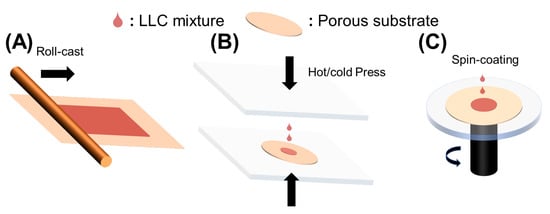
Figure 2. Methods to fabricate LLC-based TFC membranes. (A) Roll-casting; (B) hot or cold pressing; (C) Spin-coating.
The pressing method was developed to prevent solvent evaporation, as shown in Figure 2B. This method effectively reduces the possibility of water evaporation affecting TFC performances, but it leads to the infusion of the active layer into the porous support, making the active layer as thick as the support. This problem can be resolved by reducing the pore size of the porous support, improving the hydrophilicity of the substrate, decreasing the pressing temperature (need to ensure the phase formation in the specific temperature), and introducing a sacrificial layer. The sacrificial layer method has been developed to fabricate thin free-standing films and avoid infusion of LLC mesophase into the substrate. Generally, water-soluble polymers, such as polyvinyl alcohol (PVA), poly (vinylpyrrolidone) (PVP), poly (4-vinylphenol), and dextran, act as the sacrificial layers [10,12,69,70]. The main drawback of this method is water solvent absorption from the water-soluble polymer during the fabrication process, which can greatly influence the LLC phase structures. Thus, this method is only suitable for LLC systems with selective polar organic solvents.
Spin-coating (Figure 2C) is a straightforward method for producing ultrathin and uniform films, with membrane thickness controlled by solution concentration and spin speed [71]. However, for the fabrication of LLC membranes, it is crucial to maintain control over the LLC phase structure during the high spin speeds [10]. The structures and alignment [72] can be significantly altered by changes in spin speed, solvent, and even airflow direction for solvent evaporation. Moreover, a low-volatility polar organic solvent is necessary to form the LLC phase using this method.
Economic costs and life-cycle emissions are important in chemical use, fabrication, and operation needs for application [73]. Herein, economic costs and life-cycle emissions during the membrane fabrication process play important roles in future applications. From an economic cost perspective, interfacial polymerization can be a more complex and specialized process. It often requires specific equipment, chemicals, and controlled reaction conditions, which can contribute to higher upfront costs and investment in infrastructure. The cost of raw materials and the complexity of the production process can affect the overall economic feasibility of interfacial polymerization-based applications. Compared to the interfacial polymerization of PA-based TFC, the fabrication of LLC-based TFC can be more cost-effective. The three methods discussed above require less specialized equipment. Chemicals used in LLC systems are of fewer types and possess higher stability than that used in PA. Additionally, solid-like LLC precursor usually forms before membrane fabrication. This pre-polymerization process determines better reaction conditions controlled by free-radical polymerization than interfacial polymerization. From a life-cycle emission perspective, both interfacial polymerization and free-radical polymerization process produce emissions, including volatile organic compounds, greenhouse gases, and other chemical pollutants. These emissions can be mitigated by various strategies, such as optimizing reaction conditions and introducing efficient catalysts or initiators.
2.3. Performance of TFC Membranes with LLC as the Active Layer
Many LLC template membranes currently outperform the previous ones limited by trade-off, and their performances are comparable to commercial membranes (Table 1). Studies have mainly focused on building the outperformed TFCs with LLC active layers using hexagonal columnar LLC and cubic LLC. Gin and his coworkers reported a TFC with an inverted hexagonal (HII) phase as the active layer and polysulfone (Psf) UF as the substrate [34]. This HII active layer with 0.6 μm possesses a molecular separation pore size of about 1.2 nm. However, the isotropic cylindrical nanochannels without alignment presented a very low pure water flux of 0.053 Lm−2 h−1 bar−1 μm. Osuji et al. [10] reported a TFC membrane with a parallel aligned hexagonal (HI) phase active layer. In this study, a spin-coated ultrathin (200 nm) active layer provided a relatively high water flux of 2 Lm−2 h−1 bar−1 μm. The molecular weight cutoff (MWCO) of this TFC membrane is 300 Da for charged organic solutes and 600 Da for neutral organic solutes (1.2 nm PEG). The salt (MgCl2 and CaCl2) rejection of this membranes is more than 80%.
Table 1. Summary of the reported results for TFC membranes based on LLC template active layer.
| Ref. | LLC Phase | Porous Substrate | Fabrication Process | Solvent | Reactive Temp (°C) | Pore Size in Diameter (nm) | Thickness | Pure Water Flux | Rejection (%) |
|---|---|---|---|---|---|---|---|---|---|
| [34] | HII | PSf MF | Roll-casting | H2O | RT | 1.2 | 0.6 μm | 0.053 Lm−2 h−1 bar−1 μm | Na-TSO (60) Na-NpSO (73) Na-AnSO (89) Na-PySO (94) PEG600 (25.7) PEG5000 (96.1) PEG20000 (99.6) |
| [16] | QI | PE MF | Hot-pressing | H2O | 65 | 0.75 | 40 μm | 0.086 Lm−2 h−1 bar−1 μm | Ethidium Red (99.9) PEG-600 (99.9) Sucrose (99.9) Glucose (96) Glycerol (53) EG (38) NaCl (95) MgCl2 (99.3) CaCl2 (99.3) |
| [35] | QI | PE MF | Hot-pressing | H2O | 60 | 0.86 | 40 μm | 0.054 Lm−2 h−1 bar−1 μm | NaCl (94) KCl (92) MgCl2 (95) CaCl2 (96.9) Sucrose (97.9) Glucose (95) Glycerol (45) Ethylene glycol (38) |
| [9] | QI | PE MF | Hot-pressing | H2O | 65 | 0.75 | 40 μm | Using Ref. [16]’s LLC membrane. Water filtration performances in between that of commercial RO membranes and NF membranes | |
| [63] | QI | PES UF | Rod-coating | Glycerol | 70 | 0.96 | 3 μm | 0.054 Lm−2 h−1 bar−1 μm | Sucrose (97) Glucose (87) Glycerol (45) EG (24) NaCl (98) MgCl2(99) |
| [74] | QI | PES UF | Rod-coating | Glycerol | 70 | ≈1 | 3 μm | Using Ref. [63]’s LLC membrane. Anion exchange in the pores can adjust the flux with little change in rejection performance | |
| [75] | QI | PES UF | Rod-coating | Glycerol | 70 | ≈1 | 3 μm | Using Ref. [63]’s LLC membrane. TFC QI possesses a similar performance as commercial RO and NF membranes in treating hydraulic fracturing flowback water. Controllable DOC recovery can be adjusted by pH | |
| [76] | QI | PES UF | Rod-coating | Glycerol | 70 | ≈1 | 3 μm | Using Ref. [63]’s LLC membrane. The 66 h cross-flow filtration of hydraulic fracturing produced water was conducted. Better performance than NF90 in portion of organic compounds, water flux, and fouling resistance | |
| [68] | HII Lα |
PE MF (recovered) |
Hot-pressing | H2O | RT (UV-curing) + 70 (thermal-curing) |
4 nm (HII) 3 nm (Lα) |
10 μm | These LLC membranes possess better permeability and antifouling performances than commercially UF membranes. BSA rejection higher than 95% | |
| [12] | HI | PAN UF | Pressing | H2O | RT | 1–2 nm | 3–30 μm | 10 Lm−2 h−1 bar−1 μm | Methylene blue (90) Crystal violet (90) Alcian blue (90) Charged solutes (~350 Da) Neutral solutes (~4 kDa) |
| [10] | HI | PAN UF and PVDF UF | Spin-coating | Glycerol | RT | ~1 nm | ~200 nm | 2 Lm−2 h−1 bar−1 μm | PEG600(>94) Methyl orange (91) Methylene blue (95) CaCl2 and MgCl2 (>80) LiCl, NaCl, and KCl (>40) |
| [72] | HI | PVDF UF | Spin-coating | Glycerol | RT | 0.6–1.5 nm | 170–200 nm | 10–30 Lm−2 h−1 bar in water 2–8 Lm−2 h−1 bar−1 μm in methanol |
4 HI membranes possess various performances. Only list the maximum performances here. PEG 600 (100%) Acid Fuchsin 585 Da (> 95%) Methyl Orange 327 Da (100%) |
The standard for salt rejection for drinkable desalinate seawater is more than 95% [77], and the hydrated Na+ is about 0.72 nm [5,78]. However, it is very challenging to fabricate a hexagonal mesophase with continuous and uniform nanopores in a diameter range smaller than 1 nm. Gin and his coworkers reported TFC membranes based on the bi-continuous cubic (QI) phase [16,35]. The effective pore size of the active layers was found to be 0.75–0.96 nm, which allows for a 95% NaCl rejection. By replacing the water solvent with organic solvent glycerol, NaCl rejection was improved to 98% by significantly decreasing the defects in the active layer. However, the low effective pore size (<1 nm) of the QI mesophase leads to low water flux (<0.1 Lm−2 h−1 bar−1 μm) [63].
This entry is adapted from the peer-reviewed paper 10.3390/membranes13060549
This entry is offline, you can click here to edit this entry!
