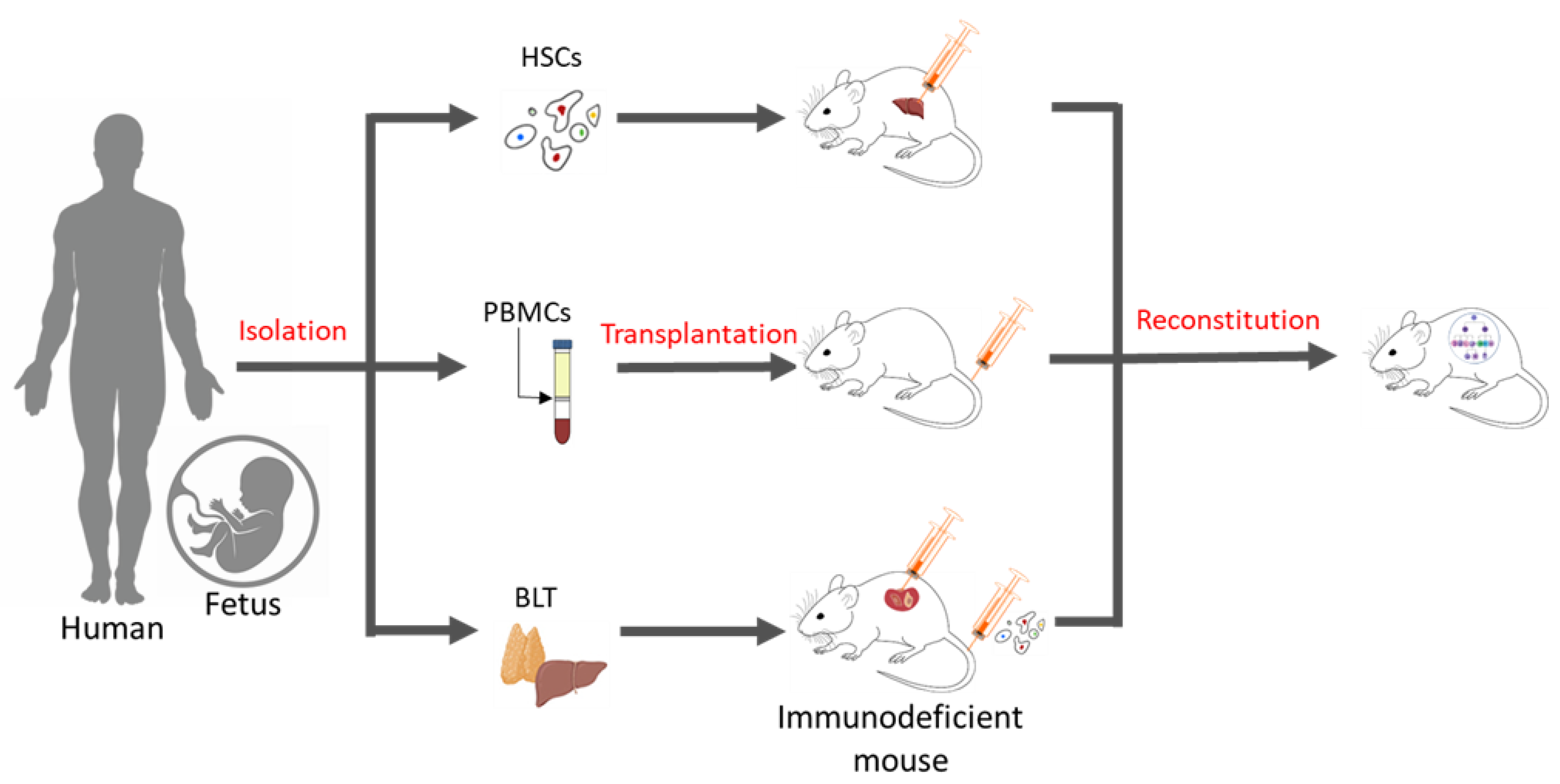
While chemotherapies remain the mainstay treatments for many cancers, the advent of new molecular techniques has opened doors for more targeted modalities towards cancer cells. Although immune checkpoint inhibitors (ICIs) have demonstrated therapeutic efficacy in treating cancer, adverse side effects related to excessive inflammation are often reported. There is a lack of clinically relevant animal models to probe the human immune response towards ICI-based interventions. Humanized mouse models have emerged as valuable tools for pre-clinical research to evaluate the efficacy and safety of immunotherapy.
- humanized mouse model
- cancer
- immunotherapy
1. Introduction
| Models | Mutations | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| SCID | Protein kinase DNA-activated catalytic peptide gene (Prkdc) | Lack humoral and cell-mediated immunity Absence of B and T cells |
Leakiness due to age Reduced lifespan Presence of murine NK cells Complementation of activity |
[4][7] |
| NOD/SCID | Signal regulatory protein alpha (Sirpa) | High affinity to human CD47Tolerance of host macrophages to the human cellsImmunological multi-dysfunction, including defective NK cell activitySupport higher levels of human engraftments | Residual NK cell activity, and some other innate compounds of the immune system, fail to develop mature monocytes, which eventually become thymic lymphomas with age |
[8][9][10][11][12][13] |
| BRG Rag1/2 |
Recombination-activating gene 1 (Rag1) or 2 (Rag2) | No leakiness Absence of functional B and T cellsLonger lifespans |
Limited lymphoid reconstitution Residual NK cell activity |
[14] |
| NOG NSG |
Interleukin-2 receptor gamma chain (IL2rγ) null phenotype and Prkdc mutation (NOD/SCID-IL2Rγ−/−) | Absence of functional receptors for cytokines like IL-2 and IL-7 Could hamper the development of host NK cells Highest rate of human cell engraftments Longer lifespans |
Weak human myeloid reconstitution Lack of human thymus tissue Radiation sensitivity |
[5][6][12][15] |
| NRG | RAG1/2 null mutation and Interleukin-2 receptor gamma chain (IL2rγ) null (NOD/RAG1/2−/−- IL2Rγ−/−) |
Improved myeloid engraftmentCan tolerate chemotherapy at higher doses | Higher radiation dose for preconditioning Lack of human thymus tissue Possible background activity |
[16] |
| FRG | Fah/Rag2/IL-2rγ | Triple knockout Expand human hepatocytes robustly Gene and cell therapy |
Remnant mouse hepatocytes Background activity |
[17][18] |
2. Humanized Mouse Models for Testing ICIs and Antibody-Based Drugs
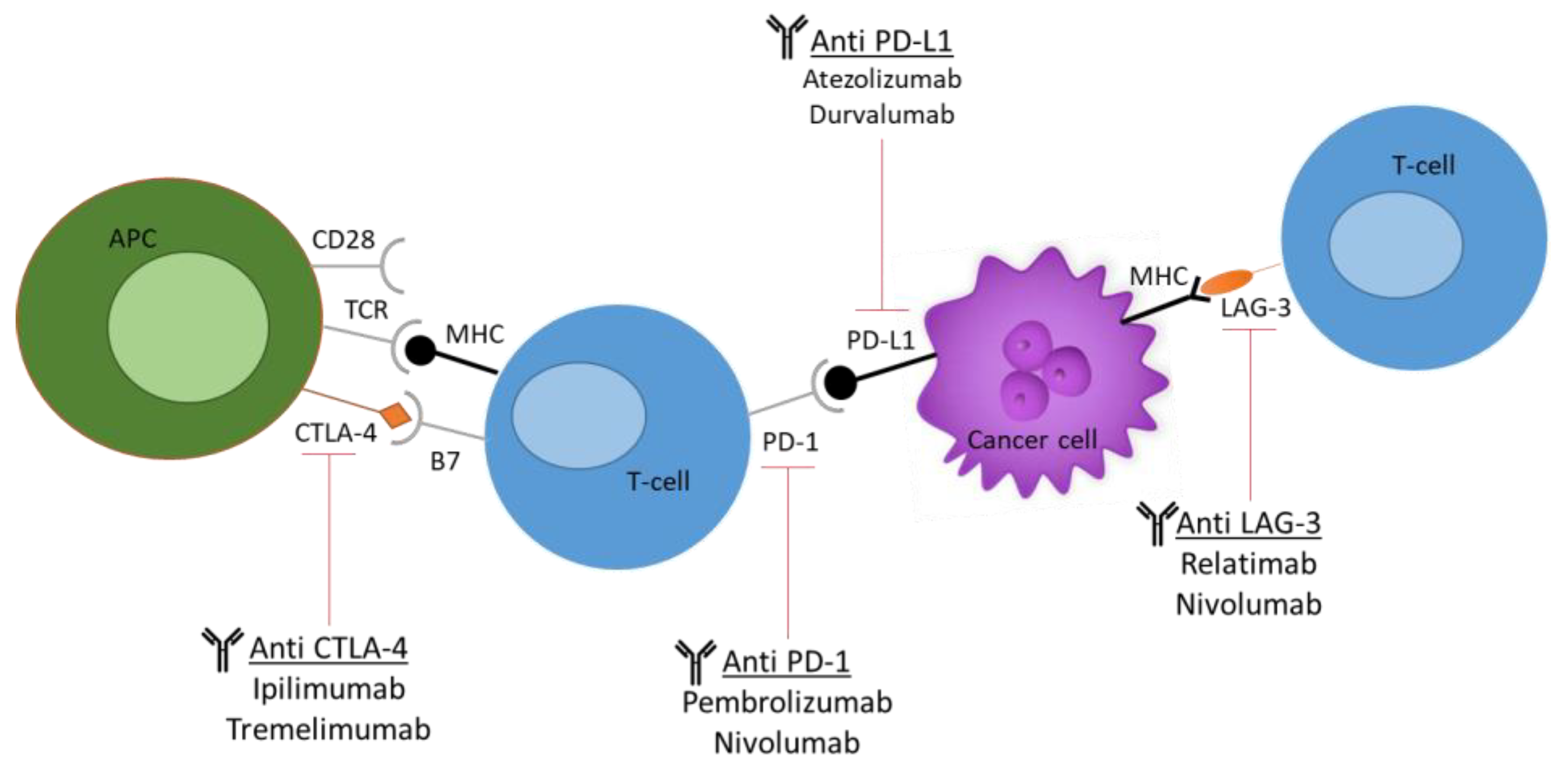
| Mouse model | Target | Safety | Efficacy | Model Description | Refs. |
|---|---|---|---|---|---|
| Colon | CD137 and PD-1 | √ | √ | CDX-model with intraperitoneal injection of PBMCs in Rag2−/−IL2Rγnull strain | [25] |
| Gastric | CD137 and PD-1 | √ | √ | PDX-model with intraperitoneal injection of PBMCs in Rag2−/−IL2Rγnull strain | [25] |
| HCC 1 | PD-1 and CTLA-4 | √ | √ | PDX-model with intrahepatic injection of human CD34+ HSCs in NSG mice | [24][33] |
| NPC 2 | PD-1 and CTLA-4 | √ | √ | PDX-model with intrahepatic injection of human CD34+ HSCs in NSG mice | [23] |
| Lymphoma | PD-1 and CTLA-4 | √ | √ | CDX-model with subcutaneous engraftment of human PBMCs in NOG mice | [37][38] |
| Sarcoma | PD-1 | √ | √ | PDX-model with intravenous injection of human CD34+ CB cells in NSG mice | [36] |
| NSCLC 3 | PD-1 | √ | √ | CDX and PDX-models with intravenous injection of human CD34+ HPSCs in NSG mice | [35][39] |
| PD-L1 | √ | √ | CDX and PDX-models with intravenous injection of either PBMCs or human CD34+ HSCs in NSG mice | [40] | |
| Bladder | PD-1 | √ | √ | PDX-model with intravenous injection of human CD34+ HPSCs in NSG mice | [35] |
| TNBC 4 | PD-1 | √ | √ | CDX and PDX-models with intravenous injection of human CD34+ HPSCs in NSG mice | [35] |
| Lung adenocarcinoma | PD-1 | √ | √ | CDX-model with intravenous injection of CD34+ human HSCs in NOG and NOG-FcγR−/− mice | [41] |
| PD-L1 | √ | √ | CDX-model with intravenous injection of cord-blood derived CD34+ human HSCs in NSG mice | [42] | |
| HNSCC 5 | PD-1 | √ | √ | CDX-model with intravenous injection of CD34+ human HSCs in NOG and NOG-FcγR−/− mice | [41] |
| Ovarian carcinoma | PD-L1 | √ | √ | CDX-model with intravenous injection of fetal liver-derived CD34+ human HSCs in NOG mice | [42] |
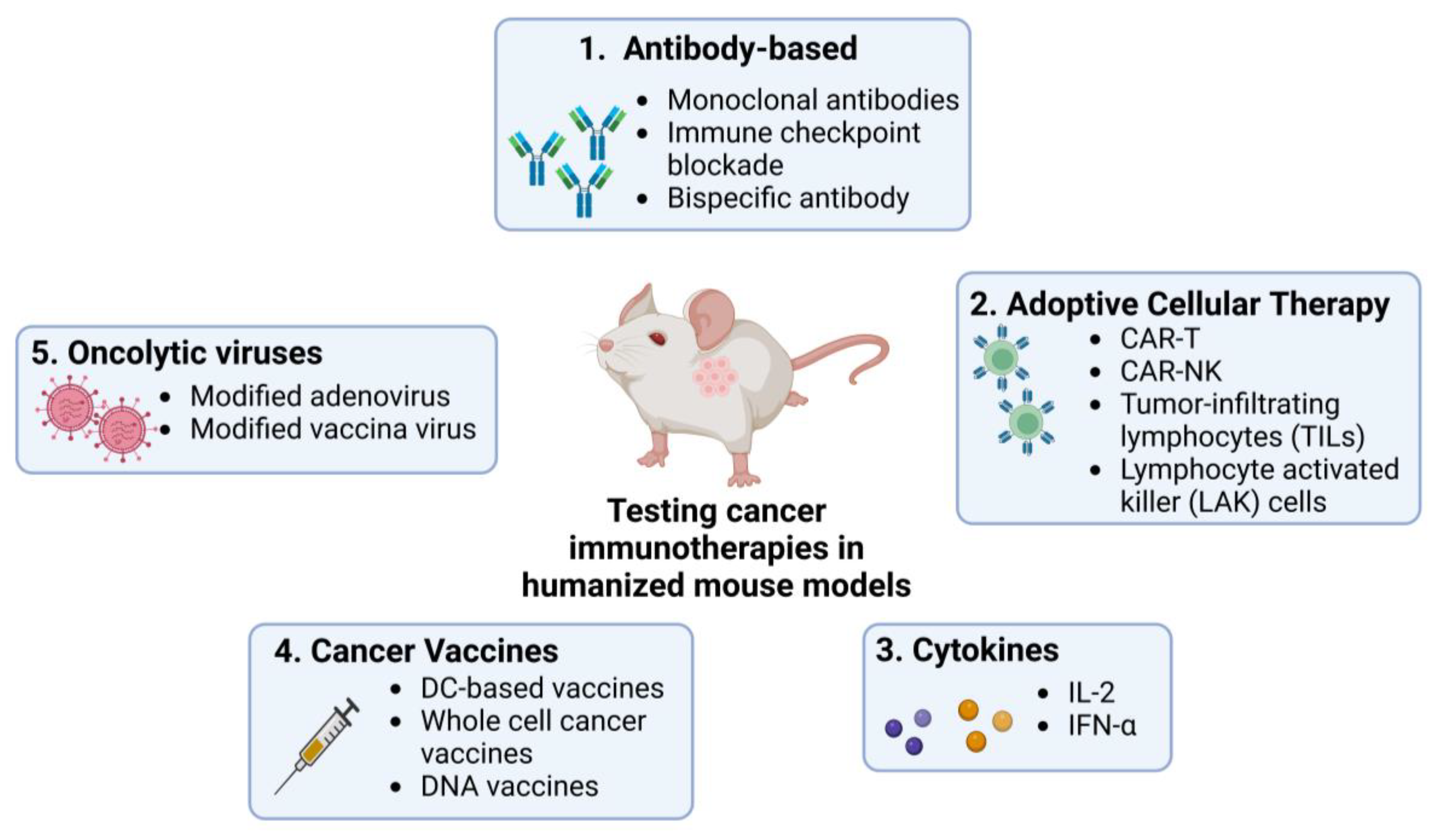
3. Safety and Efficacy Profiling of ACT in Humanized Mouse Models
3.1. CAR-T Cells
3.2. CAR-NK Cells
4. Improving Cytokine-Based Immunotherapy Using Humanized Mouse Models
5. Immune Response of Cancer Vaccines in Humanized Mouse Models
6. Targeted Tumour Lysis by Oncolytic Viruses (OVs) Using Humanized Mice
7. Combination Therapy with ICIs
| Immunotherapy | Humanized Mouse Model Application | Safety & Efficacy | Further Modifications | Limitations |
|---|---|---|---|---|
| Antibody-based | Help in discovering new targets for ICIs | Improved compared to wild type mice | Immune-transgenic models | Undefined resistance mechanisms |
| Adoptive Cell Therapy | Hints towards possible mechanisms underlying CRS | Higher safety and efficacy standards | Improved NK cell model for cytotoxic activity | CRS and neurotoxicity, limited effect on solid tumours |
| Cytokine | Gaining popularity to prevent tumour growth | Potential to improve | Fully humanized models with improved cytokine transgenic models | Lack of specificity |
| Cancer vaccines | Prophylactic and therapeutic vaccines are being established using this model | Dose escalation studies provide improved safety and efficacy | Dual humanized mouse models for specific viral cancers | Scarcity of suitable neoantigens |
| Oncolytic viruses | Discovering novel delivery systems and targeting tumour lysis | Improved safety and efficacy when translated to clinic | Dual humanized mouse models | Need for improved delivery strategies, tumour heterogeneity |
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15061600
References
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 11, 197–218.
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 1, 11.
- Flora, A. Evidence-Based Selection of the Starting Dose in First-in-Human Clinical Trials Using Humanized Mouse Models. The Jackson Laboratory. Available online: https://www.jax.org/news-and-insights/jax-blog/2022/april/evidence-based-selection-starting-dose-in-human-clinical-trials (accessed on 15 February 2023).
- Mosier, D.E.; Gulizia, R.J.; Baird, S.M.; Wilson, D.B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature 1988, 331, 256–259.
- Shultz, L.D.; Lyons, B.L.; Burzenski, L.M.; Gott, B.; Chen, X.; Chaleff, S.; Kotb, M.; Gillies, S.D.; King, M.; Mangada, J.; et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 2005, 171, 6477–6489.
- Ishikawa, F.; Yasukawa, M.; Lyons, B.; Yoshida, S.; Miyamoto, T.; Yoshimoto, G.; Watanabe, T.; Akashi, K.; Shultz, L.D.; Harada, M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor chain(null) mice. Blood 2005, 101, 1565–1573.
- Bosma, G.C.; Custer, R.P.; Bosma, M.J. A severe combined immunodeficiency mutation in the mouse. Nature 1983, 301, 527–530.
- Makino, S.; Kunimoto, K.; Muraoka, Y.; Mizushima, Y.; Katagiri, K.; Tochino, Y. Breeding of a non-obese, diabetic strain of mice. Exp. Anim. 1980, 21, 1–13.
- Mian, S.A.; Anjos-Afonso, F.; Bonnet, D. Advances in Human Immune System Mouse Models for Studying Human Hematopoiesis and Cancer Immunotherapy. Front. Immunol. 2021, 11, 619236.
- Mullen, Y. Development of the Nonobese Diabetic Mouse and Contribution of Animal Models for Understanding Type 1 Diabetes. Pancreas 2017, 41, 455–466.
- Takenaka, K.; Prasolava, T.K.; Wang, J.C.; Mortin-Toth, S.M.; Khalouei, S.; Gan, O.I.; Dick, J.E.; Danska, J.S. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat. Immunol. 2007, 1, 1313–1323.
- Shultz, L.D.; Schweitzer, P.A.; Christianson, S.W.; Gott, B.; Schweitzer, I.B.; Tennent, B.; McKenna, S.; Mobraaten, L.; Rajan, T.V.; Greiner, D.L.; et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 1995, 151, 180–191.
- Serreze, D.V.; Gaedeke, J.W.; Leiter, E.H. Hematopoietic stem-cell defects underlying abnormal macrophage development and maturation in NOD/Lt mice: Defective regulation of cytokine receptors and protein kinase C. Proc. Natl. Acad. Sci. USA 1993, 91, 9625–9629.
- Ménoret, S.; Fontanière, S.; Jantz, D.; Tesson, L.; Thinard, R.; Rémy, S.; Usal, C.; Ouisse, L.H.; Fraichard, A.; Anegon, I. Generation of Rag1-knockout immunodeficient rats and mice using engineered meganucleases. FASEB J. 2013, 21, 703–711.
- Lee, J.Y.; Han, A.R.; Lee, D.R. T Lymphocyte Development and Activation in Humanized Mouse Model. Dev. Reprod. 2019, 21, 79–92.
- Shultz, L.D.; Banuelos, S.; Lyons, B.; Samuels, R.; Burzenski, L.; Gott, B.; Lang, P.; Leif, J.; Appel, M.; Rossini, A.; et al. NOD/LtSz-Rag1nullPfpnull mice: A new model system with increased levels of human peripheral leukocyte and hematopoietic stem-cell engraftment. Transplantation 2003, 71, 1036–1042.
- Azuma, H.; Paulk, N.; Ranade, A.; Dorrell, C.; Al-Dhalimy, M.; Ellis, E.; Strom, S.; Kay, M.A.; Finegold, M.; Grompe, M. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat. Biotechnol. 2007, 21, 903–910.
- Chen, J.; Li, Y.; Lai, F.; Wang, Y.; Sutter, K.; Dittmer, U.; Ye, J.; Zai, W.; Liu, M.; Shen, F.; et al. Functional Comparison of Interferon-α Subtypes Reveals Potent Hepatitis B Virus Suppression by a Concerted Action of Interferon-α and Interferon-γ Signaling. Hepatology 2021, 71, 486–502.
- Lapidot, T.; Fajerman, Y.; Kollet, O. Immune-deficient SCID and NOD/SCID mice models as functional assays for studying normal and malignant human hematopoiesis. J. Mol. Med. 1997, 71, 664–673.
- Bosma, G.C.; Fried, M.; Custer, R.P.; Carroll, A.; Gibson, D.M.; Bosma, M.J. Evidence of functional lymphocytes in some (leaky) scid mice. J. Exp. Med. 1988, 161, 1016–1033.
- Yin, L.; Wang, X.J.; Chen, D.X.; Liu, X.N.; Wang, X.J. Humanized mouse model: A review on preclinical applications for cancer immunotherapy. Am. J. Cancer Res. 2020, 11, 4568–4584.
- Cogels, M.M.; Rouas, R.; Ghanem, G.E.; Martinive, P.; Awada, A.; Van Gestel, D.; Krayem, M. Humanized Mice as a Valuable Pre-Clinical Model for Cancer Immunotherapy Research. Front. Oncol. 2021, 11, 784947.
- Liu, W.N.; Fong, S.Y.; Tan, W.W.S.; Tan, S.Y.; Liu, M.; Cheng, J.Y.; Lim, S.; Suteja, L.; Huang, E.K.; Chan, J.K.Y.; et al. Establishment and Characterization of Humanized Mouse NPC-PDX Model for Testing Immunotherapy. Cancers 2020, 11, 1025.
- Zhao, Y.; Shuen, T.W.H.; Toh, T.B.; Chan, X.Y.; Liu, M.; Tan, S.Y.; Fan, Y.; Yang, H.; Lyer, S.G.; Bonney, G.K.; et al. Development of a new patient-derived xenograft humanised mouse model to study human-specific tumour microenvironment and immunotherapy. Gut 2018, 61, 1845–1854.
- Sanmamed, M.F.; Rodriguez, I.; Schalper, K.A.; Oñate, C.; Azpilikueta, A.; Rodriguez-Ruiz, M.E.; Morales-Kastresana, A.; Labiano, S.; Pérez-Gracia, J.L.; Martín-Algarra, S.; et al. Nivolumab and Urelumab Enhance Antitumor Activity of Human T Lymphocytes Engrafted in Rag2−/−IL2Rγnull Immunodeficient Mice. Cancer Res. 2015, 71, 3466–3478.
- Roth, M.D.; Harui, A. Human tumor infiltrating lymphocytes cooperatively regulate prostate tumor growth in a humanized mouse model. J. Immunother. Cancer 2015, 1, 12.
- Pan, C.X.; Shi, W.; Ma, A.H.; Zhang, H.; Lara, P.; Keck, J.G.; Palucka, K.; Airhart, S.D.; White, R.D. Humanized mice (humice) carrying patient-derived xenograft (PDX) as a platform to develop immunotherapy in bladder cancer (BCa). Clin. Oncol. 2017, 31 (Suppl. S6), 381.
- Morton, J.J.; Bird, G.; Keysar, S.B.; Astling, D.P.; Lyons, T.R.; Anderson, R.T.; Glogowska, M.J.; Estes, P.; Eagles, J.R.; Le, P.N.; et al. XactMice: Humanizing mouse bone marrow enables microenvironment reconstitution in a patient-derived xenograft model of head and neck cancer. Oncogene 2016, 31, 290–300.
- Ito, A.; Ishida, T.; Yano, H.; Inagaki, A.; Suzuki, S.; Sato, F.; Takino, H.; Mori, F.; Ri, M.; Kusumoto, S.; et al. Defucosylated anti-CCR4 monoclonal antibody exercises potent ADCC-mediated antitumor effect in the novel tumor-bearing humanized NOD/Shi-scid, IL-2Rgamma(null) mouse model. Cancer Immunol. Immunother. 2009, 51, 1195–1206.
- Tsoneva, D.; Minev, B.; Frentzen, A.; Zhang, Q.; Wege, A.K.; Szalay, A.A. Humanized Mice with Subcutaneous Human Solid Tumors for Immune Response Analysis of Vaccinia Virus-Mediated Oncolysis. Mol. Ther. Oncolytics 2017, 1, 41–61.
- Schupp, J.; Christians, A.; Zimmer, N.; Gleue, L.; Jonuleit, H.; Helm, M.; Tuettenberg, A. In-Depth Immune-Oncology Studies of the Tumor Microenvironment in a Humanized Melanoma Mouse Model. Int. J. Mol. Sci. 2021, 21, 1011.
- Franzin, R.; Netti, G.S.; Spadaccino, F.; Porta, C.; Gesualdo, L.; Stallone, G.; Castellano, G.; Ranieri, E. The Use of Immune Checkpoint Inhibitors in Oncology and the Occurrence of AKI: Where Do We Stand? Front. Immunol. 2020, 11, 574271.
- Zhao, Y.; Wang, J.; Liu, W.N.; Fong, S.Y.; Shuen, T.W.H.; Liu, M.; Harden, S.; Tan, S.Y.; Cheng, J.Y.; Tan, W.W.S.; et al. Analysis and Validation of Human Targets and Treatments Using a Hepatocellular Carcinoma-Immune Humanized Mouse Model. Hepatology 2021, 71, 1395–1410.
- He, Q.F.; Xu, Y.; Li, J.; Huang, Z.M.; Li, X.H.; Wang, X. CD8+ T-cell exhaustion in cancer: Mechanisms and new area for cancer immunotherapy. Brief Funct. Genom. 2019, 11, 99–106.
- Wang, M.; Yao, L.C.; Cheng, M.; Cai, D.; Martinek, J.; Pan, C.X.; Shi, W.; Ma, A.H.; De Vere White, R.W.; Airhart, S.; et al. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J. 2018, 31, 1537–1549.
- Choi, B.; Lee, J.S.; Kim, S.J.; Hong, D.; Park, J.B.; Lee, K.Y. Anti-tumor effects of anti-PD-1 antibody, pembrolizumab, in humanized NSG PDX mice xenografted with dedifferentiated liposarcoma. Cancer Lett. 2020, 471, 56–69.
- Donnou, S.; Galand, C.; Touitou, V.; Sautès-Fridman, C.; Fabry, Z.; Fisson, S. Murine models of B-cell lymphomas: Promising tools for designing cancer therapies. Adv. Hematol. 2012, 2011, 701704.
- Ma, S.D.; Xu, X.; Jones, R.; Delecluse, H.J.; Zumwalde, N.A.; Sharma, A.; Gumperz, J.E.; Kenney, S.C. PD-1/CTLA-4 Blockade Inhibits Epstein-Barr Virus-Induced Lymphoma Growth in a Cord Blood Humanized-Mouse Model. PLoS Pathog. 2016, 12, e1005642.
- Qiao, T.; Xiong, Y.; Feng, Y.; Guo, W.; Zhou, Y.; Zhao, J.; Jiang, T.; Shi, C.; Han, Y. Inhibition of LDH-A by Oxamate Enhances the Efficacy of Anti-PD-1 Treatment in an NSCLC Humanized Mouse Model. Front. Oncol. 2021, 11, 632364.
- Lin, S.; Huang, G.; Cheng, L.; Li, Z.; Xiao, Y.; Deng, Q.; Jiang, Y.; Li, B.; Lin, S.; Wang, S.; et al. Establishment of peripheral blood mononuclear cell-derived humanized lung cancer mouse models for studying efficacy of PD-L1/PD-1 targeted immunotherapy. MAbs 2018, 11, 1301–1311.
- Katano, I.; Hanazawa, A.; Otsuka, I.; Yamaguchi, T.; Mochizuki, M.; Kawai, K.; Ito, R.; Goto, M.; Kagawa, T.; Takahashi, T. Development of a novel humanized mouse model for improved evaluation of in vivo anti-cancer effects of anti-PD-1 antibody. Sci. Rep. 2021, 11, 21087.
- Li, Y.; Carpenito, C.; Wang, G.; Surguladze, D.; Forest, A.; Malabunga, M.; Murphy, M.; Zhang, Y.; Sonyi, A.; Chin, D.; et al. Discovery and preclinical characterization of the antagonist anti-PD-L1 monoclonal antibody LY3300054. J. Immunother. Cancer 2018, 1, 31, Erratum in J. Immunother. Cancer 2018, 1, 45.
- Rosenberg, S.A.; Dudley, M.E. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr. Opin. Immunol. 2009, 21, 233–240.
- Vanegas, Y.M.; Mohty, R.; Gadd, M.E.; Luo, Y.; Aljurf, M.; Qin, H.; Kharfan-Dabaja, M.A. CAR-T cell Therapies for B-cell Lymphoid Malignancies: Identifying Targets Beyond CD19. Hematol. Oncol. Stem Cell Ther. 2022, 11, 81–93.
- Eshhar, Z.; Waks, T.; Gross, G.; Schindler, D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA 1993, 91, 720–724.
- Marofi, F.; Achmad, H.; Bokov, D.; Abdelbasset, W.K.; Alsadoon, Z.; Chupradit, S.; Suksatan, W.; Shariatzadeh, S.; Hasanpoor, Z.; Yazdanifar, M.; et al. Hurdles to breakthrough in CAR T cell therapy of solid tumors. Stem Cell Res. Ther. 2022, 11, 140.
- Havard, R.; Stephens, D.M. Anti-CD19 chimeric antigen receptor T cell therapies: Harnessing the power of the immune system to fight diffuse large b cell lymphoma. Curr. Hematol. Malig. Rep. 2018, 11, 534–542.
- Ali, S.A.; Shi, V.; Maric, I.; Wang, M.; Stroncek, D.F.; Rose, J.J.; Brudno, J.N.; Stetler-Stevenson, M.; Feldman, S.A.; Hansen, B.G.; et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016, 121, 1688–1700.
- Giavridis, T.; van der Stegen, S.J.C.; Eyquem, J.; Hamieh, M.; Piersigilli, A.; Sadelain, M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 2018, 21, 731–738.
- Diorio, C.; Murray, R.; Naniong, M.; Barrera, L.; Camblin, A.; Chukinas, J.; Coholan, L.; Edwards, A.; Fuller, T.; Gonzales, C.; et al. Cytosine base editing enables quadruple-edited allogeneic CART cells for T-ALL. Blood 2022, 141, 619–629.
- Choi, B.D.; Yu, X.; Castano, A.P.; Darr, H.; Henderson, D.B.; Bouffard, A.A.; Larson, R.C.; Scarfo, I.; Bailey, S.R.; Gerhard, G.M.; et al. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J. Immunother. Cancer 2019, 1, 304.
- Jin, C.H.; Xia, J.; Rafiq, S.; Huang, X.; Hu, Z.; Zhou, X.; Brentjens, R.J.; Yang, Y.G. Modeling anti-CD19 CAR T cell therapy in humanized mice with human immunity and autologous leukemia. EBioMedicine 2019, 31, 173–181.
- Li, H.; Song, W.; Li, Z.; Zhang, M. Preclinical and clinical studies of CAR-NK-cell therapies for malignancies. Front. Immunol. 2022, 11, 992232.
- Liu, W.N.; So, W.Y.; Harden, S.L.; Fong, S.Y.; Wong, M.X.Y.; Tan, W.W.S.; Tan, S.Y.; Ong, J.K.L.; Rajarethinam, R.; Liu, M.; et al. Successful targeting of PD-1/PD-L1 with chimeric antigen receptor-natural killer cells and nivolumab in a humanized mouse cancer model. Sci. Adv. 2022, 8, eadd1187.
- Liu, E.; Tong, Y.; Dotti, G.; Shaim, H.; Savoldo, B.; Mukherjee, M.; Orange, J.; Wan, X.; Lu, X.; Reynolds, A.; et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2018, 31, 520–531.
- Chulpanova, D.S.; Kitaeva, K.V.; Green, A.R.; Rizvanov, A.A.; Solovyeva, V.V. Molecular Aspects and Future Perspectives of Cytokine-Based Anti-cancer Immunotherapy. Front. Cell Dev. Biol. 2020, 1, 402.
- Jiang, T.; Zhou, C.; Ren, S. Role of IL-2 in cancer immunotherapy. Oncoimmunology 2016, 5, e1163462.
- Lotze, M.T.; Frana, L.W.; Sharrow, S.O.; Robb, R.J.; Rosenberg, S.A. In vivo administration of purified human interleukin 2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin 2. J. Immunol. 1985, 131, 157–166.
- Lotze, M.T.; Matory, Y.L.; Ettinghausen, S.E.; Rayner, A.A.; Sharrow, S.O.; Seipp, C.A.; Custer, M.C.; Rosenberg, S.A. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J. Immunol. 1985, 131, 2865–2875.
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 191, 5451–5458.
- Rosenberg, S.A.; Lotze, M.T.; Muul, L.M.; Chang, A.E.; Avis, F.P.; Leitman, S.; Linehan, W.M.; Robertson, C.N.; Lee, R.E.; Rubin, J.T.; et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N. Engl. J. Med. 1987, 311, 889–897.
- Bankert, R.B.; Balu-Iyer, S.V.; Odunsi, K.; Shultz, L.D.; Kelleher RJJr Barnas, J.L.; Simpson-Abelson, M.; Parsons, R.; Yokota, S.J. Humanized mouse model of ovarian cancer recapitulates patient solid tumor progression, ascites formation, and metastasis. PLoS ONE 2011, 6, e24420.
- Stanley, M. Tumour virus vaccines: Hepatitis B virus and human papillomavirus. Philos. Trans. R Soc. Lond. B Biol. Sci. 2017, 371, 20160268.
- Roudko, V.; Greenbaum, B.; Bhardwaj, N. Computational Prediction and Validation of Tumor-Associated Neoantigens. Front. Immunol. 2020, 11, 27.
- Spranger, S.; Frankenberger, B.; Schendel, D.J. NOD/scid IL-2Rg(null) mice: A preclinical model system to evaluate human dendritic cell-based vaccine strategies in vivo. J. Transl. Med. 2012, 11, 30.
- Jhajharia, S.; Lai, F.; Low, H.B.; Purushotorman, K.; Shunmuganathan, B.D.; Chan, C.E.Z.; Hammond, R.; Netter, H.J.; Chen, Q.; Lim, S.G.; et al. Defining the specificity and function of a human neutralizing antibody for Hepatitis B virus. NPJ Vaccines 2022, 1, 121.
- Lawler, S.E.; Speranza, M.C.; Cho, C.F.; Chiocca, E.A. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol. 2017, 1, 841–849.
- Yu, Y.A.; Shabahang, S.; Timiryasova, T.M.; Zhang, Q.; Beltz, R.; Gentschev, I.; Goebel, W.; Szalay, A.A. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat. Biotechnol. 2004, 21, 313–320.
- Zhang, Q.; Yu, Y.A.; Wang, E.; Chen, N.; Danner, R.L.; Munson, P.J.; Marincola, F.M.; Szalay, A.A. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res. 2007, 61, 10038–10046.
- Dey, M.; Yu, D.; Kanojia, D.; Li, G.; Sukhanova, M.; Spencer, D.A.; Pituch, K.C.; Zhang, L.; Han, Y.; Ahmed, A.U.; et al. Intranasal Oncolytic Virotherapy with CXCR4-Enhanced Stem Cells Extends Survival in Mouse Model of Glioma. Stem Cell Rep. 2016, 1, 471–482.
- Minev, B.; Kohrt, H.; Kilinc, M.; Chen, N.; Feng, A.; Pessian, M.; Geissinger, U.; Haefner, E.; Tsoneva, D.; Bozhilov, K.; et al. Combination immunotherapy with oncolytic vaccinia virus and checkpoint inhibitor following local tumor irradiation. J. Immunother. Cancer 2014, 2 (Suppl. S3), P112.
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 1, 86.
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 361, 122–133, Erratum in N. Engl. J. Med. 2018, 371, 2185.
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. RELATIVITY-047 Investigators. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 381, 24–34.
- Sinha, D.; Srihari, S.; Beckett, K.; Le Texier, L.; Solomon, M.; Panikkar, A.; Ambalathingal, G.R.; Lekieffre, L.; Crooks, P.; Rehan, S.; et al. ‘Off-the-shelf’ allogeneic antigen-specific adoptive T-cell therapy for the treatment of multiple EBV-associated malignancies. J. Immunother. Cancer 2021, 9, e001608.
- Jiang, Z.; Jiang, X.; Chen, S.; Lai, Y.; Wei, X.; Li, B.; Lin, S.; Wang, S.; Wu, Q.; Liang, Q.; et al. Anti-GPC3-CAR T Cells Suppress the Growth of Tumor Cells in Patient-Derived Xenografts of Hepatocellular Carcinoma. Front. Immunol. 2017, 1, 690.
- Huang, R.Y.; Francois, A.; McGray, A.R.; Miliotto, A.; Odunsi, K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology 2016, 6, e1249561.
- Capasso, A.; Lang, J.; Pitts, T.M.; Jordan, K.R.; Lieu, C.H.; Davis, S.L.; Diamond, J.R.; Kopetz, S.; Barbee, J.; Peterson, J.; et al. Characterization of immune responses to anti-PD-1 mono and combination immunotherapy in hematopoietic humanized mice implanted with tumor xenografts. J. Immunother. Cancer 2019, 1, 37.
- Ha, W.; Sevim-Nalkiran, H.; Zaman, A.M.; Matsuda, K.; Khasraw, M.; Nowak, A.K.; Chung, L.; Baxter, R.C.; McDonald, K.L. Ibudilast sensitizes glioblastoma to temozolomide by targeting Macrophage Migration Inhibitory Factor (MIF). Sci. Rep. 2019, 1, 2905.
