Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Japanese encephalitis virus (JEV), a zoonotic flavivirus, is principally transmitted by hematophagous mosquitoes, continually between susceptible animals and incidentally from those animals to humans.
- Japanese encephalitis virus
- flavivirus
- pathogenesis
1. Introduction
Japanese encephalitis virus (JEV) is a mosquito-borne arbovirus taxonomically belonging to the genus Flavivirus in the family Flaviviridae [1]. Of the 53 classified species of known flaviviruses, JEV is related to other medically important mosquito-borne flaviviruses to various genetic and antigenic extents, with a much closer relationship being noted with West Nile (WNV), St. Louis encephalitis (SLEV), and Murray Valley encephalitis viruses than with Zika (ZIKV), dengue (DENV), and yellow fever (YFV) viruses [2][3]. JEV circulates in nature by both horizontal transmission among susceptible animal hosts, primarily through the bite of culicine mosquito vectors (e.g., Culex tritaeniorhynchus) [4][5], and vertical transmission from female mosquitoes to their offspring through the transovarial infection of developing eggs [6][7]. Of many susceptible animals, certain vertebrates, such as suids (e.g., domestic pigs) and avians (e.g., wading birds), are especially relevant for the incidental transmission of JEV to the human population [8][9][10]. Experimentally, JEV can also be transmitted in the absence of mosquito vectors through several non-vector-borne routes in animals such as rodents, pigs, bats, and/or squirrel monkeys: contact transmission [11][12][13][14], aerosol transmission [11][15], transplacental transmission [16][17][18][19][20], and artificial insemination [21][22]. In both humans and animals, JEV causes Japanese encephalitis (JE), formerly called Type B or Japanese B encephalitis [5][23], which is an acute encephalitis syndrome that potentially leads to severe brain damage or death [24][25]. In Japan, where JE was first recognized, the disease is believed to have arisen in the “summer encephalitis” season before the 20th century began, although the first recorded outbreak occurred in 1924, involving >6000 human cases with a case fatality of ~60% [26][27]. About a decade after this historic outbreak, a filterable agent from the brain of a fatal human JE case was demonstrated to be able to produce the disease in monkeys. In 1935, JEV was first isolated from the brain of a deceased patient, and the isolate was designated as the Nakayama strain [28]. Despite the fact that JEV has been known as the cause of JE for nearly a century, it is still a neglected pathogen that continues to be a major public health challenge [29].
JEV is the most common cause of viral encephalitis in the Asia-Pacific region [30][31][32][33], with the reported boundaries of viral activity on the north being much of China and the eastern China-Russia borderlands [34][35][36][37][38][39][40], on the south being Papua New Guinea [41][42] and a northern part of Australia [43][44][45][46][47][48][49], on the east being Guam [50][51][52] and Saipan [53][54], and on the west being much of India [55][56] and a southeastern part of Pakistan [57][58]. This region contains ~25 countries, including the top two most populous countries (i.e., China and India) of the world and several of the most densely populated countries (e.g., Singapore and Bangladesh), putting far more than half of the global population at risk of JEV infection [59]. Additionally, some cases of JEV infection were found in birds [60][61] and mosquitoes [62] collected in Italy during the first decade of the 21st century, which was the first time that the virus was detected outside the Asia-Pacific region, albeit with no human JE outbreaks reported as of yet. In 2016, however, a case of co-infection with JEV and YFV was identified unexpectedly in an Angolan resident with no out-of-country travel history [63]. This recent epidemiological data indicates that JEV is no longer confined to the Asia-Pacific region, and is continuously expanding its activity over new territories in Europe and Africa, with possible spread to the Americas in the very near future [64][65]. Hence, JEV is an emerging pathogen that has the potential to spread across the globe, now more than ever [66][67][68].
The annual incidence of JE presents two main epidemiological patterns [69][70], intrinsically aligned with the regional climate that affects the population size, density, and distribution of both mosquito vectors and animal hosts involved in JEV transmission [71][72]. In tropical regions, JEV is “endemic,” causing sporadic outbreaks nearly all year round at low frequencies, with peaks in the rainy season affecting all young under age 15 due to lack of immunity to the virus. In subtropical and temperate regions, on the other hand, JEV is “epidemic”, causing seasonal outbreaks exclusively during the summer and early fall and affecting all ages, with a bimodal distribution peaking in young children and the elderly because of the lack and the fading of JEV-specific immunity, respectively. Since its emergence, JEV has evolved into five divergent genotypes (G1–G5) presumably at the center of the Asia-Pacific region (i.e., Indonesia and Malaysia), from which one or more genotypes have dispersed to other areas of the region [73]. Historically, G1, G2, and G3 have all been circulated broadly at various times and frequencies in the Asia-Pacific region, except the India-Nepal-Sri Lanka area in which only G1 and G3 have been detected, and the Australia-Papua New Guinea area in which only G1 and G2 have been found [74][75][76][77][78][79][80][81][82][83]. Of particular note, G3 was the most prevalent in Asia until the 1990s, but since then, it has slowly been replaced by G1 [74][75][76][77][78][79][80]; yet, G3 has recently appeared for the first time outside the Asia-Pacific region in Italy [60][61][62] and Angola [63]. Unlike the three aforementioned genotypes, G4 has been located only in the Indonesia-Malaysia area [84], and G5 has been limited to the China-Japan-Korea area apart from the Indonesia-Malaysia area [85][86][87][88]. Within the five genotypes, it is noted that the sequence divergence between their genomes at the nucleotide (nt) and amino acid (aa) levels reaches up to ~20% and ~10%, respectively [85]; however, their biological differences in viral transmissibility, pathogenicity, and immunogenicity have not yet been fully understood.
2. The Virus
JEV is an enveloped virus with a linear, single-stranded, positive-sense RNA genome [89]. The virion consists of an inner nucleocapsid composed of the genomic RNA and multiple capsid C:C homodimers [90], and an outer shell composed of two surface proteins M and E arranged into 90 E:M:M:E heterotetramers [91] in a herringbone pattern on the viral envelope membrane (Figure 1, top panel). The genomic RNA is ~10,977 nt long (Figure 1, bottom panel) and has three functional parts [92]: (1) an ~95-nt untranslated region (UTR) with a type 1 cap structure attached at the 5’ end [93], (2) an ~10,299-nt central open reading frame (ORF) with a -1 ribosomal frameshift signal located at positions nt 3551-3630 [94], and (3) an ~583-nt UTR with no poly(A) tail at the 3’ end [95]. Each of the 5’ and 3’ UTRs, as well as the ~100-nt 5’-terminal region of the ORF, contains a network of cis-acting RNA elements [96][97][98] defined by the primary, secondary, and tertiary structures that regulate the translation and replication of the viral genomic RNA [99][100][101][102]. The single long ORF encodes two precursor polyproteins [103]: (i) the full-length ppC–NS5, which undergoes co- and post-translational proteolytic cleavages to yield three structural (C, prM/M, and E) and seven nonstructural (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) proteins; and (ii) the frameshift-derived truncated ppC–NS1’, which also goes through the same proteolysis as does ppC–NS5 to produce the three aforementioned structural proteins and NS1’, a 52-aa longer isoform of NS1 as a result of its C-terminal extension that includes the 9-aa N-terminal segment of NS2A before the frameshift and a 43-aa unique peptide synthesized from the new reading frame afterwards [92][104][105]. Of these 10 mature proteins, the following two are enzymes, each containing multiple catalytic activities [106][107][108][109]: (a) NS3, 619 aa in length, has serine protease activity in the ~168-aa N-terminal region [110] and helicase, nucleoside 5’-triphosphatase, and RNA 5’-triphosphatase activities in the ~438-aa C-terminal region [111], and (b) NS5, 905 aa in length, has methyltransferase and guanylyltransferase activities in the ~265-aa N-terminal region and RNA-dependent RNA polymerase activity in the ~630-aa C-terminal region [112]. In a nutshell, the three structural proteins are required for the formation of an infectious virion [113][114][115], and the seven nonstructural proteins are engaged in multiple steps of viral replication (i.e., polyprotein processing [116], RNA replication [117][118][119], and particle morphogenesis [120][121]) and in a variety of host cell responses to viral replication [122][123][124][125]. With regard to JEV pathogenesis, the viral E protein has been the target of extensive study since it acts as the viral receptor interacting with an ill-defined cellular component(s) on susceptible host cells for viral entry [126][127][128]. In addition, a recent study has suggested that a secreted form of the viral NS1 protein specifically binds to brain endothelial cells and alters their permeability, causing brain-specific vascular leakage that contributes to the pathogenesis of JE [129].
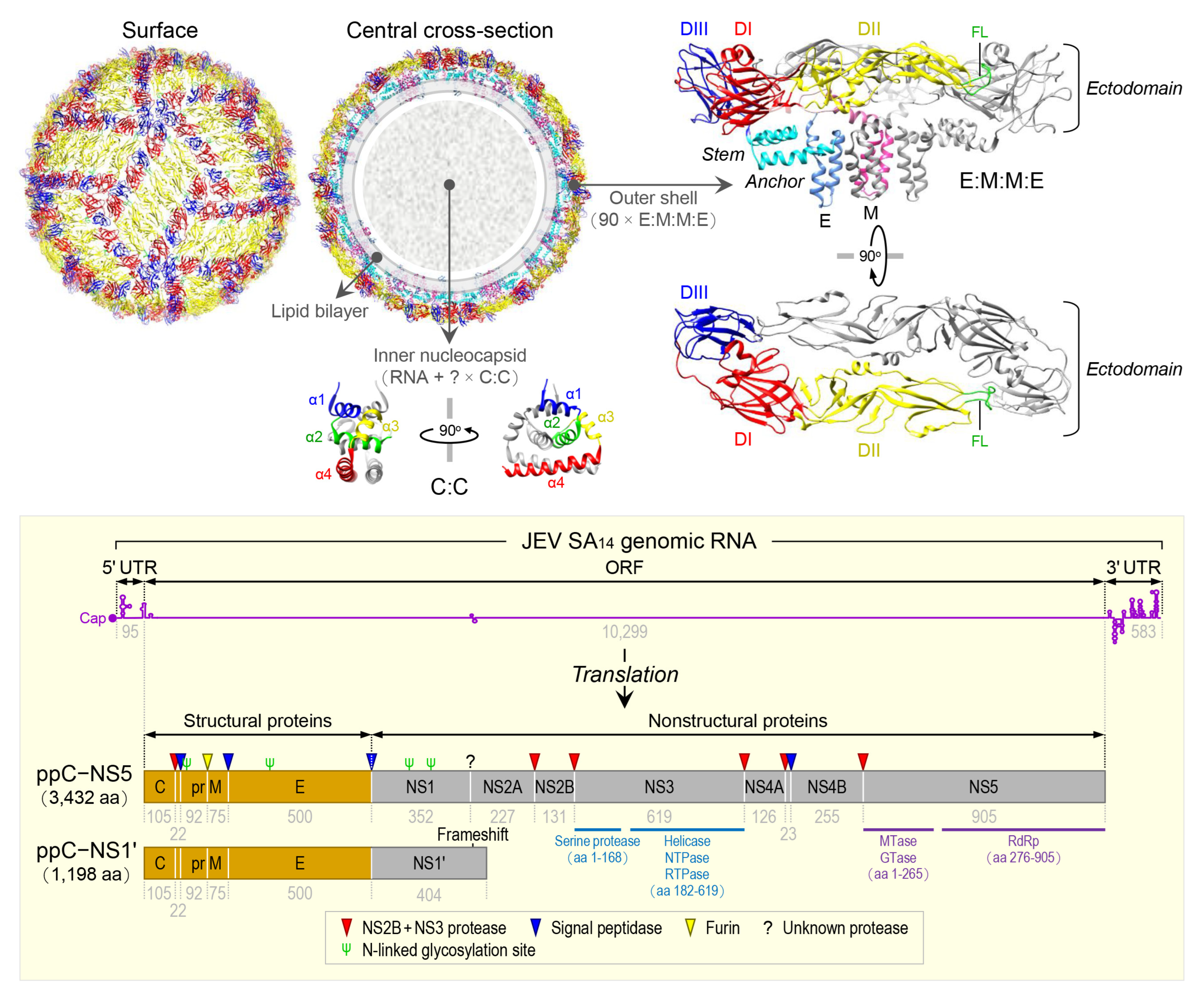
Figure 1. Virion structure, genome organization, and gene expression of JEV. The top panel shows the high-resolution cryo-electron microscopy structure of JEV strain P3 [91]. The virion contains multiple copies of three proteins: capsid (C), envelope (E), and membrane (M). The C monomer is a cytosolic protein containing four helices (α1, blue; α2, green; α3, yellow; and α4, red). The E monomer is an integral membrane protein comprising three topologically distinct parts: an N-terminal ectodomain, which has three structural domains (DI, red; DII, yellow; and DIII, blue) with the fusion loop (FL, green) positioned at the distal end of DII; a stem (cyan), which has three non-membrane-spanning helices; and a C-terminal anchor (cornflower blue), which has two membrane-spanning helices. The M monomer is also an integral membrane protein comprising three topologically distinct parts: an N-terminal extension containing an unstructured peptide fragment (pink), a non-membrane-spanning helix (hot pink), and a C-terminal anchor containing two membrane-spanning helices (deep pink). The bottom panel depicts the genome organization and gene expression of JEV strain SA14 [92]. The genome is a capped but unpolyadenylated plus-strand RNA, with a single long open reading frame (ORF) flanked by short, highly structured 5’ and 3’ untranslated regions (UTRs). The ORF is translated into two polyprotein precursors, both of which are cleaved by viral and cellular proteases, as indicated, to produce three structural (orange) and seven nonstructural (gray) proteins. Of these proteins, two are multifunctional enzymes: First, NS3 has serine protease, helicase, NTPase, and RTPase activities. Second, NS5 has MTase, GTase, and RdRp activities. Four N-linked glycosylation sites are marked, one in the pr portion of prM, one in E, and two in NS1/1’.
3. Clinical Features in Humans
For symptomatic JEV infection, it generally takes 5–15 days with a median of 8.4 days to show clinical signs/symptoms after contraction of the virus, but in some cases, may have a longer incubation time of 3–4 weeks [130]. The clinical outcomes vary significantly [131][132] from mild self-limiting undifferentiated illnesses (e.g., fever) to severe life-threatening nervous system diseases (e.g., encephalitis) [133][134][135][136].
3.1. Central Nervous System Disorders
JE, a neuroinflammatory disease, is the most serious outcome of JEV infection attacking neurons and other cell types in the central nervous system (CNS) [24]. JE almost always starts with nonspecific symptoms, such as lethargy, fever, chills, coryza, and diarrhea for a few days prior to the onset of neurological manifestations, such as headache, vomiting, impaired consciousness, altered mental status, and seizures [137][138][139][140][141][142][143][144][145]. Seizures tend to occur more frequently in young children [141][142][146]. Critically ill JE patients may present a Parkinsonian syndrome with expressionless faces, tremors, and hypertonia linked to the damage of the basal ganglia in the brain [147][148] or a polio-like syndrome with acute flaccid paralysis caused by destruction of the anterior horn cells in the spinal cord [149][150][151][152][153][154]. Other movement disorders observed in JE patients include opisthotonus, dystonia, hemiparesis, choreoathetosis, orofacial dyskinesias, and gaze palsy [138][139][140][143][155]. Occasionally, JE patients develop neuroimmunological complications, such as anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis caused by an antibody-mediated autoimmune reaction to the NMDARs in the brain [156][157][158], acute transverse myelitis characterized by an inflammation in the spinal cord often damaging the myelin sheath [159][160], and acute disseminated encephalomyelitis noted in general by an inflammatory demyelination affecting the CNS [161][162]. In addition, non-neurological manifestations are also associated with JE, which include gastric hemorrhage, thrombocytopenia, liver dysfunction, hepatomegaly, splenomegaly, and potentially pulmonary edema [138][163][164]. Poor prognosis for JE is indicated by persistent/recurrent fever, prolonged/repeated seizures, poor plantar reflexes, severe lung and kidney problems, high JEV antigens in cerebrospinal fluid (CSF), and low anti-JEV IgM antibodies in CSF and serum [141][143][165][166][167][168]. Even after recovery, a large portion of JE survivors have neuropsychiatric sequelae, such as memory loss, intellectual disability, language disorders, emotional disorders, behavioral disorders, movement disorders, and ocular motor disorders [169][170][171][172][173].
3.2. Peripheral Nervous System Disorders
Limited clinical studies have suggested that JEV infection may induce Guillain–Barré syndrome (GBS) [174][175][176], a neuroimmunological disease caused by our own immune system primarily marring the myelin sheaths and axons of nerves of the peripheral nervous system (PNS) [177]. To date, the most informative study conducted with 47 JEV-positive GBS patients has shown that the disease usually begins with febrile illness, as seen during the prodromal phase of JE, which then progresses to neurological complications, such as headache, disturbed consciousness, paresthesia, impaired reflexes, flaccid paralysis, and breathing problems [175]. In the same study, a follow-up examination with 17 of the initial 47 GBS patients at eight months after hospital discharge found that they still experienced neuromuscular complications, such as limb muscle weakness and atrophy, incontinence, and hoarse voice [175]. Although its causal relationship with GBS requires further investigation, JEV appears to be able to trigger the PNS-damaging GBS. In line with this notion, other flaviviruses have recently been documented to be a cause of GBS, which include WNV [178][179], ZIKV [180][181], and DENV [182][183].
3.3. Reproductive Disorders
It is conceivable that pregnant women infected with JEV may pass the virus to their fetus, based on the recent findings that during gestation, ZIKV can cross the placenta, infect the unborn offspring, and thus cause miscarriage or a variety of birth defects, collectively designated as congenital Zika syndrome, such as microcephaly [184]. However, little is known about the possible adverse effects of JEV infection during pregnancy. Until now, only a few human cases of congenital JEV infection have been reported, which result in either abortions or the deliveries of a healthy child [185][186][187][188], possibly depending on the time of infection during gestation [187]. In pregnant sows, on the other hand, it has been well established that congenital JEV infection causes reproductive losses, such as mummified, stillborn, and abnormal piglets even when they are born alive [189][190][191][192][193]. Therefore, there is a clear difference in the clinical outcome of congenital JEV infections between humans and pigs, both of which are highly susceptible to the virus. It may be worth investigating a possible causal link between JEV infection and pregnancy complications in humans, and if there is a link, then its potential effects on fetal growth in general and fetal brain development in particular, should be studied.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens12050715
References
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV virus taxonomy profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3.
- Gould, E.A.; de Lamballerie, X.; Zanotto, P.M.; Holmes, E.C. Origins, evolution, and vector/host coadaptations within the genus Flavivirus. Adv. Virus Res. 2003, 59, 277–314.
- Mackenzie, J.S.; Barrett, A.D.; Deubel, V. The Japanese encephalitis serological group of flaviviruses: A brief introduction to the group. Curr. Top. Microbiol. Immunol. 2002, 267, 1–10.
- Pearce, J.C.; Learoyd, T.P.; Langendorf, B.J.; Logan, J.G. Japanese encephalitis: The vectors, ecology and potential for expansion. J. Travel Med. 2018, 25, S16–S26.
- Rosen, L. The natural history of Japanese encephalitis virus. Annu. Rev. Microbiol. 1986, 40, 395–414.
- Rosen, L.; Tesh, R.B.; Lien, J.C.; Cross, J.H. Transovarial transmission of Japanese encephalitis virus by mosquitoes. Science 1978, 199, 909–911.
- Rosen, L.; Lien, J.C.; Shroyer, D.A.; Baker, R.H.; Lu, L.C. Experimental vertical transmission of Japanese encephalitis virus by Culex tritaeniorhynchus and other mosquitoes. Am. J. Trop. Med. Hyg. 1989, 40, 548–556.
- Mansfield, K.L.; Hernandez-Triana, L.M.; Banyard, A.C.; Fooks, A.R.; Johnson, N. Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet. Microbiol. 2017, 201, 85–92.
- Lord, J.S.; Gurley, E.S.; Pulliam, J.R. Rethinking Japanese encephalitis virus transmission: A framework for implicating host and vector species. PLoS Negl. Trop. Dis. 2015, 9, e0004074.
- Oliveira, A.R.S.; Cohnstaedt, L.W.; Cernicchiaro, N. Japanese encephalitis virus: Placing disease vectors in the epidemiologic triad. Ann. Entomol. Soc. Am. 2018, 111, 295–303.
- Chai, C.; Palinski, R.; Xu, Y.; Wang, Q.; Cao, S.; Geng, Y.; Zhao, Q.; Wen, Y.; Huang, X.; Yan, Q.; et al. Aerosol and contact transmission following intranasal infection of mice with Japanese encephalitis virus. Viruses 2019, 11, 87.
- Lyons, A.C.; Huang, Y.S.; Park, S.L.; Ayers, V.B.; Hettenbach, S.M.; Higgs, S.; McVey, D.S.; Noronha, L.; Hsu, W.W.; Vanlandingham, D.L. Shedding of Japanese encephalitis virus in oral fluid of infected swine. Vector Borne Zoonotic Dis. 2018, 18, 469–474.
- Garcia-Nicolas, O.; Braun, R.O.; Milona, P.; Lewandowska, M.; Dijkman, R.; Alves, M.P.; Summerfield, A. Targeting of the nasal mucosa by Japanese encephalitis virus for non-vector-borne transmission. J. Virol. 2018, 92, e01091-18.
- Ricklin, M.E.; Garcia-Nicolas, O.; Brechbuhl, D.; Python, S.; Zumkehr, B.; Nougairede, A.; Charrel, R.N.; Posthaus, H.; Oevermann, A.; Summerfield, A. Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nat. Commun. 2016, 7, 10832.
- Larson, E.W.; Dominik, J.W.; Slone, T.W. Aerosol stability and respiratory infectivity of Japanese B encephalitis virus. Infect. Immun. 1980, 30, 397–401.
- Mathur, A.; Arora, K.L.; Chaturvedi, U.C. Transplacental Japanese encephalitis virus (JEV) infection in mice during consecutive pregnancies. J. Gen. Virol. 1982, 59, 213–217.
- Chapagain, S.; Pal Singh, P.; Le, K.; Safronetz, D.; Wood, H.; Karniychuk, U. Japanese encephalitis virus persists in the human reproductive epithelium and porcine reproductive tissues. PLoS Negl. Trop. Dis. 2022, 16, e0010656.
- Mathur, A.; Arora, K.L.; Chaturvedi, U.C. Congenital infection of mice with Japanese encephalitis virus. Infect. Immun. 1981, 34, 26–29.
- Park, S.L.; Huang, Y.S.; Vanlandingham, D.L. Re-examining the importance of pigs in the transmission of Japanese encephalitis virus. Pathogens 2022, 11, 575.
- Sulkin, S.E.; Sims, R.; Allen, R. Studies of arthropod-borne virus infections in Chiroptera. II. Experiments with Japanese B and St. Louis encephalitis viruses in the gravid bat. Evidence of transplacental transmission. Am. J. Trop. Med. Hyg. 1964, 13, 475–481.
- Maes, D.; Van Soom, A.; Appeltant, R.; Arsenakis, I.; Nauwynck, H. Porcine semen as a vector for transmission of viral pathogens. Theriogenology 2016, 85, 27–38.
- Althouse, G.C.; Rossow, K. The potential risk of infectious disease dissemination via artificial insemination in swine. Reprod. Domest. Anim. 2011, 46 (Suppl. S2), 64–67.
- Solomon, T. Control of Japanese encephalitis--within our grasp? N. Engl. J. Med. 2006, 355, 869–871.
- Turtle, L.; Solomon, T. Japanese encephalitis—The prospects for new treatments. Nat. Rev. Neurol. 2018, 14, 298–313.
- Misra, U.K.; Kalita, J. Overview: Japanese encephalitis. Prog. Neurobiol. 2010, 91, 108–120.
- Miyake, M. The pathology of Japanese encephalitis. Bull. World Health Organ. 1964, 30, 153–160.
- Tiroumourougane, S.V.; Raghava, P.; Srinivasan, S. Japanese viral encephalitis. Postgrad. Med. J. 2002, 78, 205–215.
- Yun, S.I.; Lee, Y.M. Japanese encephalitis virus: Molecular biology and vaccine development. In Molecular Biology of the Flavivirus; Kalitzky, M., Borowski, P., Eds.; Horizon Scientific Press: Norwich, UK, 2006; pp. 225–271.
- Kline, K.; McCarthy, J.S.; Pearson, M.; Loukas, A.; Hotez, P.J. Neglected tropical diseases of Oceania: Review of their prevalence, distribution, and opportunities for control. PLoS Negl. Trop. Dis. 2013, 7, e1755.
- Endy, T.P.; Nisalak, A. Japanese encephalitis virus: Ecology and epidemiology. Curr. Top. Microbiol. Immunol. 2002, 267, 11–48.
- Erlanger, T.E.; Weiss, S.; Keiser, J.; Utzinger, J.; Wiedenmayer, K. Past, present, and future of Japanese encephalitis. Emerg. Infect. Dis. 2009, 15, 1–7.
- Van den Hurk, A.F.; Ritchie, S.A.; Mackenzie, J.S. Ecology and geographical expansion of Japanese encephalitis virus. Annu. Rev. Entomol. 2009, 54, 17–35.
- Mackenzie, J.S.; Williams, D.T. The zoonotic flaviviruses of southern, south-eastern and eastern Asia, and Australasia: The potential for emergent viruses. Zoonoses Public Health 2009, 56, 338–356.
- Li, Y.X.; Li, M.H.; Fu, S.H.; Chen, W.X.; Liu, Q.Y.; Zhang, H.L.; Da, W.; Hu, S.L.; Mu, S.D.; Bai, J.; et al. Japanese encephalitis, Tibet, China. Emerg. Infect. Dis. 2011, 17, 934–936.
- Zheng, Y.; Li, M.; Wang, H.; Liang, G. Japanese encephalitis and Japanese encephalitis virus in mainland China. Rev. Med. Virol. 2012, 22, 301–322.
- Wang, L.H.; Fu, S.H.; Wang, H.Y.; Liang, X.F.; Cheng, J.X.; Jing, H.M.; Cai, G.L.; Li, X.W.; Ze, W.Y.; Lv, X.J.; et al. Japanese encephalitis outbreak, Yuncheng, China, 2006. Emerg. Infect. Dis. 2007, 13, 1123–1125.
- Gao, X.; Li, X.; Li, M.; Fu, S.; Wang, H.; Lu, Z.; Cao, Y.; He, Y.; Zhu, W.; Zhang, T.; et al. Vaccine strategies for the control and prevention of Japanese encephalitis in Mainland China, 1951–2011. PLoS Negl. Trop. Dis. 2014, 8, e3015.
- Xufang, Y.; Huanyu, W.; Shihong, F.; Xiaoyan, G.; Shuye, Z.; Chunting, L.; Minghua, L.; Yougang, Z.; Guodong, L. Etiological spectrum of clinically diagnosed Japanese encephalitis cases reported in Guizhou Province, China, in 2006. J. Clin. Microbiol. 2010, 48, 1343–1349.
- Yin, Z.; Wang, H.; Yang, J.; Luo, H.; Li, Y.; Hadler, S.C.; Sandhu, H.S.; Fischer, M.; Jiang, Y.; Zhang, Z.; et al. Japanese encephalitis disease burden and clinical features of Japanese encephalitis in four cities in the People’s Republic of China. Am. J. Trop. Med. Hyg. 2010, 83, 766–773.
- Zhang, H.; Luo, H.; Ur Rehman, M.; Nabi, F.; Li, K.; Lan, Y.; Huang, S.; Zhang, L.; Mehmood, K.; Shahzad, M.; et al. Evidence of JEV in Culex tritaeniorhynchus and pigs from high altitude regions of Tibet, China. J. Vector Borne Dis. 2017, 54, 69–73.
- Johansen, C.A.; van den Hurk, A.F.; Ritchie, S.A.; Zborowski, P.; Nisbet, D.J.; Paru, R.; Bockarie, M.J.; Macdonald, J.; Drew, A.C.; Khromykh, T.I.; et al. Isolation of Japanese encephalitis virus from mosquitoes (Diptera: Culicidae) collected in the Western Province of Papua New Guinea, 1997–1998. Am. J. Trop. Med. Hyg. 2000, 62, 631–638.
- Hanson, J.P.; Taylor, C.T.; Richards, A.R.; Smith, I.L.; Boutlis, C.S. Japanese encephalitis acquired near Port Moresby: Implications for residents and travellers to Papua New Guinea. Med. J. Aust. 2004, 181, 282–283.
- Hanna, J.N.; Ritchie, S.A.; Phillips, D.A.; Lee, J.M.; Hills, S.L.; van den Hurk, A.F.; Pyke, A.T.; Johansen, C.A.; Mackenzie, J.S. Japanese encephalitis in north Queensland, Australia, 1998. Med. J. Aust. 1999, 170, 533–536.
- Hanna, J.N.; Ritchie, S.A.; Phillips, D.A.; Shield, J.; Bailey, M.C.; Mackenzie, J.S.; Poidinger, M.; McCall, B.J.; Mills, P.J. An outbreak of Japanese encephalitis in the Torres Strait, Australia, 1995. Med. J. Aust. 1996, 165, 256–260.
- Ritchie, S.A.; Phillips, D.; Broom, A.; Mackenzie, J.; Poidinger, M.; van den Hurk, A. Isolation of Japanese encephalitis virus from Culex annulirostris in Australia. Am. J. Trop. Med. Hyg. 1997, 56, 80–84.
- Ritchie, S.A.; Rochester, W. Wind-blown mosquitoes and introduction of Japanese encephalitis into Australia. Emerg. Infect. Dis. 2001, 7, 900–903.
- Van den Hurk, A.F.; Montgomery, B.L.; Northill, J.A.; Smith, I.L.; Zborowski, P.; Ritchie, S.A.; Mackenzie, J.S.; Smith, G.A. Short report: The first isolation of Japanese encephalitis virus from mosquitoes collected from mainland Australia. Am. J. Trop. Med. Hyg. 2006, 75, 21–25.
- Van den Hurk, A.F.; Ritchie, S.A.; Johansen, C.A.; Mackenzie, J.S.; Smith, G.A. Domestic pigs and Japanese encephalitis virus infection, Australia. Emerg. Infect. Dis. 2008, 14, 1736–1738.
- Van den Hurk, A.F.; Johansen, C.A.; Zborowski, P.; Phillips, D.A.; Pyke, A.T.; Mackenzie, J.S.; Ritchie, S.A. Flaviviruses isolated from mosquitoes collected during the first recorded outbreak of Japanese encephalitis virus on Cape York Peninsula, Australia. Am. J. Trop. Med. Hyg. 2001, 64, 125–130.
- Hammon, W.M.; Tigertt, W.D.; Sather, G.E.; Berge, T.O.; Meiklejohn, G. Epidemiologic studies of concurrent virgin epidemics of Japanese B encephalitis and of mumps on Guam, 1947–1948, with subsequent observations including dengue, through 1957. Am. J. Trop. Med. Hyg. 1958, 7, 441–467.
- Edgren, D.C.; Palladino, V.S.; Arnold, A. Japanese B and mumps encephalitis: A clinicopathological report of simultaneous outbreaks on the island of Guam. Am. J. Trop. Med. Hyg. 1958, 7, 471–480.
- Reeves, W.C.; Rudnick, A. A survey of the mosquitoes of Guam in two periods in 1948 and 1949 and its epidemiological implications. Am. J. Trop. Med. Hyg. 1951, 31, 633–658.
- Paul, W.S.; Moore, P.S.; Karabatsos, N.; Flood, S.P.; Yamada, S.; Jackson, T.; Tsai, T.F. Outbreak of Japanese encephalitis on the island of Saipan, 1990. J. Infect. Dis. 1993, 167, 1053–1058.
- Mitchell, C.J.; Savage, H.M.; Smith, G.C.; Flood, S.P.; Castro, L.T.; Roppul, M. Japanese encephalitis on Saipan: A survey of suspected mosquito vectors. Am. J. Trop. Med. Hyg. 1993, 48, 585–590.
- Kanagasabai, K.; Joshua, V.; Ravi, M.; Sabarinathan, R.; Kirubakaran, B.K.; Ramachandran, V.; Murhekar, M.V. Epidemiology of Japanese encephalitis in India: Analysis of laboratory surveillance data, 2014–2017. J. Infect. 2018, 76, 317–320.
- Baruah, A.; Hazarika, R.A.; Barman, N.N.; Islam, S.; Gulati, B.R. Mosquito abundance and pig seropositivity as a correlate of Japanese encephalitis in human population in Assam, India. J. Vector Borne Dis. 2018, 55, 291–296.
- Igarashi, A.; Tanaka, M.; Morita, K.; Takasu, T.; Ahmed, A.; Ahmed, A.; Akram, D.S.; Waqar, M.A. Detection of West Nile and Japanese encephalitis viral genome sequences in cerebrospinal fluid from acute encephalitis cases in Karachi, Pakistan. Microbiol. Immunol. 1994, 38, 827–830.
- Darwish, M.A.; Hoogstraal, H.; Roberts, T.J.; Ahmed, I.P.; Omar, F. A sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 442–445.
- Wang, H.; Liang, G. Epidemiology of Japanese encephalitis: Past, present, and future prospects. Ther. Clin. Risk Manag. 2015, 11, 435–448.
- Platonov, A.; Rossi, G.; Karan, L.; Mironov, K.; Busani, L.; Rezza, G. Does the Japanese encephalitis virus (JEV) represent a threat for human health in Europe? Detection of JEV RNA sequences in birds collected in Italy. Eurosurveillance 2012, 17, 20241.
- Preziuso, S.; Mari, S.; Mariotti, F.; Rossi, G. Detection of Japanese encephalitis virus in bone marrow of healthy young wild birds collected in 1997–2000 in Central Italy. Zoonoses Public Health 2018, 65, 798–804.
- Ravanini, P.; Huhtamo, E.; Ilaria, V.; Crobu, M.G.; Nicosia, A.M.; Servino, L.; Rivasi, F.; Allegrini, S.; Miglio, U.; Magri, A.; et al. Japanese encephalitis virus RNA detected in Culex pipiens mosquitoes in Italy. Eurosurveillance 2012, 17, 20221.
- Simon-Loriere, E.; Faye, O.; Prot, M.; Casademont, I.; Fall, G.; Fernandez-Garcia, M.D.; Diagne, M.M.; Kipela, J.M.; Fall, I.S.; Holmes, E.C.; et al. Autochthonous Japanese encephalitis with yellow fever coinfection in Africa. N. Engl. J. Med. 2017, 376, 1483–1485.
- Nett, R.J.; Campbell, G.L.; Reisen, W.K. Potential for the emergence of Japanese encephalitis virus in California. Vector Borne Zoonotic Dis. 2009, 9, 511–517.
- Oliveira, A.R.S.; Cohnstaedt, L.W.; Noronha, L.E.; Mitzel, D.; McVey, D.S.; Cernicchiaro, N. Perspectives regarding the risk of introduction of the Japanese encephalitis virus in the United States. Front. Vet. Sci. 2020, 7, 48.
- Mackenzie, J.S.; Gubler, D.J.; Petersen, L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004, 10, S98–S109.
- Dash, A.P.; Bhatia, R.; Sunyoto, T.; Mourya, D.T. Emerging and re-emerging arboviral diseases in Southeast Asia. J. Vector Borne Dis. 2013, 50, 77–84.
- Mackenzie, J.S.; Johansen, C.A.; Ritchie, S.A.; van den Hurk, A.F.; Hall, R.A. Japanese encephalitis as an emerging virus: The emergence and spread of Japanese encephalitis virus in Australasia. Curr. Top. Microbiol. Immunol. 2002, 267, 49–73.
- Burke, D.S.; Leake, C.J. Japanese encephalitis. In The Arboviruses: Epidemiology and Ecology; Monath, T.P., Ed.; CRC Press: Boca Raton, FL, USA, 1988; pp. 63–92.
- Vaughn, D.W.; Hoke, C.H., Jr. The epidemiology of Japanese encephalitis: Prospects for prevention. Epidemiol. Rev. 1992, 14, 197–221.
- Le Flohic, G.; Porphyre, V.; Barbazan, P.; Gonzalez, J.P. Review of climate, landscape, and viral genetics as drivers of the Japanese encephalitis virus ecology. PLoS Negl. Trop. Dis. 2013, 7, e2208.
- Miller, R.H.; Masuoka, P.; Klein, T.A.; Kim, H.C.; Somer, T.; Grieco, J. Ecological niche modeling to estimate the distribution of Japanese encephalitis virus in Asia. PLoS Negl. Trop. Dis. 2012, 6, e1678.
- Solomon, T.; Ni, H.; Beasley, D.W.; Ekkelenkamp, M.; Cardosa, M.J.; Barrett, A.D. Origin and evolution of Japanese encephalitis virus in southeast Asia. J. Virol. 2003, 77, 3091–3098.
- Pan, X.L.; Liu, H.; Wang, H.Y.; Fu, S.H.; Liu, H.Z.; Zhang, H.L.; Li, M.H.; Gao, X.Y.; Wang, J.L.; Sun, X.H.; et al. Emergence of genotype I of Japanese encephalitis virus as the dominant genotype in Asia. J. Virol. 2011, 85, 9847–9853.
- Gao, X.; Liu, H.; Li, X.; Fu, S.; Cao, L.; Shao, N.; Zhang, W.; Wang, Q.; Lu, Z.; Lei, W.; et al. Changing geographic distribution of Japanese encephalitis virus genotypes, 1935–2017. Vector Borne Zoonotic Dis. 2019, 19, 35–44.
- Gao, X.; Liu, H.; Wang, H.; Fu, S.; Guo, Z.; Liang, G. Southernmost Asia is the source of Japanese encephalitis virus (genotype 1) diversity from which the viruses disperse and evolve throughout Asia. PLoS Negl. Trop. Dis. 2013, 7, e2459.
- Schuh, A.J.; Ward, M.J.; Leigh Brown, A.J.; Barrett, A.D. Dynamics of the emergence and establishment of a newly dominant genotype of Japanese encephalitis virus throughout Asia. J. Virol. 2014, 88, 4522–4532.
- Gao, X.; Liu, H.; Li, M.; Fu, S.; Liang, G. Insights into the evolutionary history of Japanese encephalitis virus (JEV) based on whole-genome sequences comprising the five genotypes. Virol. J. 2015, 12, 43.
- Wang, H.Y.; Takasaki, T.; Fu, S.H.; Sun, X.H.; Zhang, H.L.; Wang, Z.X.; Hao, Z.Y.; Zhang, J.K.; Tang, Q.; Kotaki, A.; et al. Molecular epidemiological analysis of Japanese encephalitis virus in China. J. Gen. Virol. 2007, 88, 885–894.
- Nga, P.T.; Parquet, M.D.C.; Cuong, V.D.; Ma, S.P.; Hasebe, F.; Inoue, S.; Makino, Y.; Takagi, M.; Nam, V.S.; Morita, K. Shift in Japanese encephalitis virus (JEV) genotype circulating in northern Vietnam: Implications for frequent introductions of JEV from Southeast Asia to East Asia. J. Gen. Virol. 2004, 85, 1625–1631.
- Williams, D.T.; Wang, L.F.; Daniels, P.W.; Mackenzie, J.S. Molecular characterization of the first Australian isolate of Japanese encephalitis virus, the FU strain. J. Gen. Virol. 2000, 81, 2471–2480.
- Schuh, A.J.; Li, L.; Tesh, R.B.; Innis, B.L.; Barrett, A.D. Genetic characterization of early isolates of Japanese encephalitis virus: Genotype II has been circulating since at least 1951. J. Gen. Virol. 2010, 91, 95–102.
- Schuh, A.J.; Tesh, R.B.; Barrett, A.D. Genetic characterization of Japanese encephalitis virus genotype II strains isolated from 1951 to 1978. J. Gen. Virol. 2011, 92, 516–527.
- Schuh, A.J.; Guzman, H.; Tesh, R.B.; Barrett, A.D. Genetic diversity of Japanese encephalitis virus isolates obtained from the Indonesian archipelago between 1974 and 1987. Vector Borne Zoonotic Dis. 2013, 13, 479–488.
- Li, M.H.; Fu, S.H.; Chen, W.X.; Wang, H.Y.; Guo, Y.H.; Liu, Q.Y.; Li, Y.X.; Luo, H.M.; Da, W.; Duo Ji, D.Z.; et al. Genotype V Japanese encephalitis virus is emerging. PLoS Negl. Trop. Dis. 2011, 5, e1231.
- Li, M.H.; Fu, S.H.; Chen, W.X.; Wang, H.Y.; Cao, Y.X.; Liang, G.D. Molecular characterization of full-length genome of Japanese encephalitis virus genotype V isolated from Tibet, China. Biomed. Environ. Sci. 2014, 27, 231–239.
- Takhampunya, R.; Kim, H.C.; Tippayachai, B.; Kengluecha, A.; Klein, T.A.; Lee, W.J.; Grieco, J.; Evans, B.P. Emergence of Japanese encephalitis virus genotype V in the Republic of Korea. Virol. J. 2011, 8, 449.
- Kim, H.; Cha, G.W.; Jeong, Y.E.; Lee, W.G.; Chang, K.S.; Roh, J.Y.; Yang, S.C.; Park, M.Y.; Park, C.; Shin, E.H. Detection of Japanese encephalitis virus genotype V in Culex orientalis and Culex pipiens (Diptera: Culicidae) in Korea. PLoS ONE 2015, 10, e0116547.
- Yun, S.I.; Lee, Y.M. Japanese encephalitis virus. In Encyclopedia of Virology, 4th ed.; Bamford, D., Zuckerman, M., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 583–597.
- Poonsiri, T.; Wright, G.S.A.; Solomon, T.; Antonyuk, S.V. Crystal structure of the Japanese encephalitis virus capsid protein. Viruses 2019, 11, 623.
- Wang, X.; Li, S.H.; Zhu, L.; Nian, Q.G.; Yuan, S.; Gao, Q.; Hu, Z.; Ye, Q.; Li, X.F.; Xie, D.Y.; et al. Near-atomic structure of Japanese encephalitis virus reveals critical determinants of virulence and stability. Nat. Commun. 2017, 8, 14.
- Yun, S.I.; Song, B.H.; Polejaeva, I.A.; Davies, C.J.; White, K.L.; Lee, Y.M. Comparison of the live-attenuated Japanese encephalitis vaccine SA14-14-2 strain with its pre-attenuated virulent parent SA14 strain: Similarities and differences in vitro and in vivo. J. Gen. Virol. 2016, 97, 2575–2591.
- Dong, H.; Fink, K.; Zust, R.; Lim, S.P.; Qin, C.F.; Shi, P.Y. Flavivirus RNA methylation. J. Gen. Virol. 2014, 95, 763–778.
- Firth, A.E.; Atkins, J.F. A conserved predicted pseudoknot in the NS2A-encoding sequence of West Nile and Japanese encephalitis flaviviruses suggests NS1′ may derive from ribosomal frameshifting. Virol. J. 2009, 6, 14.
- Hahn, C.S.; Hahn, Y.S.; Rice, C.M.; Lee, E.; Dalgarno, L.; Strauss, E.G.; Strauss, J.H. Conserved elements in the 3′ untranslated region of flavivirus RNAs and potential cyclization sequences. J. Mol. Biol. 1987, 198, 33–41.
- Yun, S.I.; Choi, Y.J.; Song, B.H.; Lee, Y.M. 3′ Cis-acting elements that contribute to the competence and efficiency of Japanese encephalitis virus genome replication: Functional importance of sequence duplications, deletions, and substitutions. J. Virol. 2009, 83, 7909–7930.
- Song, B.H.; Yun, S.I.; Choi, Y.J.; Kim, J.M.; Lee, C.H.; Lee, Y.M. A complex RNA motif defined by three discontinuous 5-nucleotide-long strands is essential for flavivirus RNA replication. RNA 2008, 14, 1791–1813.
- Yun, S.I.; Lee, Y.M. Zika virus: An emerging flavivirus. J. Microbiol. 2017, 55, 204–219.
- Brinton, M.A.; Basu, M. Functions of the 3′ and 5′ genome RNA regions of members of the genus Flavivirus. Virus Res. 2015, 206, 108–119.
- Ng, W.C.; Soto-Acosta, R.; Bradrick, S.S.; Garcia-Blanco, M.A.; Ooi, E.E. The 5′ and 3′ untranslated regions of the flaviviral genome. Viruses 2017, 9, 137.
- Villordo, S.M.; Carballeda, J.M.; Filomatori, C.V.; Gamarnik, A.V. RNA structure duplications and flavivirus host adaptation. Trends Microbiol. 2016, 24, 270–283.
- Zeng, M.; Duan, Y.; Zhang, W.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Zhao, X.; Yang, Q.; Wu, Y.; et al. Universal RNA secondary structure insight into mosquito-borne flavivirus (MBFV) cis-acting RNA biology. Front. Microbiol. 2020, 11, 473.
- Kim, J.K.; Kim, J.M.; Song, B.H.; Yun, S.I.; Yun, G.N.; Byun, S.J.; Lee, Y.M. Profiling of viral proteins expressed from the genomic RNA of Japanese encephalitis virus using a panel of 15 region-specific polyclonal rabbit antisera: Implications for viral gene expression. PLoS ONE 2015, 10, e0124318.
- Melian, E.B.; Hinzman, E.; Nagasaki, T.; Firth, A.E.; Wills, N.M.; Nouwens, A.S.; Blitvich, B.J.; Leung, J.; Funk, A.; Atkins, J.F.; et al. NS1’ of flaviviruses in the Japanese encephalitis virus serogroup is a product of ribosomal frameshifting and plays a role in viral neuroinvasiveness. J. Virol. 2010, 84, 1641–1647.
- Ye, Q.; Li, X.F.; Zhao, H.; Li, S.H.; Deng, Y.Q.; Cao, R.Y.; Song, K.Y.; Wang, H.J.; Hua, R.H.; Yu, Y.X.; et al. A single nucleotide mutation in NS2A of Japanese encephalitis live vaccine virus (SA14-14-2) ablates NS1′ formation and contributes to attenuation. J. Gen. Virol. 2012, 93, 1959–1964.
- Assenberg, R.; Mastrangelo, E.; Walter, T.S.; Verma, A.; Milani, M.; Owens, R.J.; Stuart, D.I.; Grimes, J.M.; Mancini, E.J. Crystal structure of a novel conformational state of the flavivirus NS3 protein: Implications for polyprotein processing and viral replication. J. Virol. 2009, 83, 12895–12906.
- Luo, D.; Xu, T.; Hunke, C.; Gruber, G.; Vasudevan, S.G.; Lescar, J. Crystal structure of the NS3 protease-helicase from dengue virus. J. Virol. 2008, 82, 173–183.
- El Sahili, A.; Lescar, J. Dengue virus non-structural protein 5. Viruses 2017, 9, 91.
- Ferrero, D.S.; Ruiz-Arroyo, V.M.; Soler, N.; Uson, I.; Guarne, A.; Verdaguer, N. Supramolecular arrangement of the full-length Zika virus NS5. PLoS Pathog. 2019, 15, e1007656.
- Weinert, T.; Olieric, V.; Waltersperger, S.; Panepucci, E.; Chen, L.; Zhang, H.; Zhou, D.; Rose, J.; Ebihara, A.; Kuramitsu, S.; et al. Fast native-SAD phasing for routine macromolecular structure determination. Nat. Methods 2015, 12, 131–133.
- Yamashita, T.; Unno, H.; Mori, Y.; Tani, H.; Moriishi, K.; Takamizawa, A.; Agoh, M.; Tsukihara, T.; Matsuura, Y. Crystal structure of the catalytic domain of Japanese encephalitis virus NS3 helicase/nucleoside triphosphatase at a resolution of 1.8 A. Virology 2008, 373, 426–436.
- Lu, G.; Gong, P. Crystal structure of the full-length Japanese encephalitis virus NS5 reveals a conserved methyltransferase-polymerase interface. PLoS Pathog. 2013, 9, e1003549.
- Harrison, S.C. Viral membrane fusion. Nat. Struct. Mol. Biol. 2008, 15, 690–698.
- Hasan, S.S.; Sevvana, M.; Kuhn, R.J.; Rossmann, M.G. Structural biology of Zika virus and other flaviviruses. Nat. Struct. Mol. Biol. 2018, 25, 13–20.
- Oliveira, E.R.A.; Mohana-Borges, R.; de Alencastro, R.B.; Horta, B.A.C. The flavivirus capsid protein: Structure, function and perspectives towards drug design. Virus Res. 2017, 227, 115–123.
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990, 44, 649–688.
- Brinton, M.A. Replication cycle and molecular biology of the West Nile virus. Viruses 2013, 6, 13–53.
- Paranjape, S.M.; Harris, E. Control of dengue virus translation and replication. Curr. Top. Microbiol. Immunol. 2010, 338, 15–34.
- Bollati, M.; Alvarez, K.; Assenberg, R.; Baronti, C.; Canard, B.; Cook, S.; Coutard, B.; Decroly, E.; de Lamballerie, X.; Gould, E.A.; et al. Structure and functionality in flavivirus NS-proteins: Perspectives for drug design. Antivir. Res. 2010, 87, 125–148.
- Diosa-Toro, M.; Prasanth, K.R.; Bradrick, S.S.; Garcia Blanco, M.A. Role of RNA-binding proteins during the late stages of flavivirus replication cycle. Virol. J. 2020, 17, 60.
- Murray, C.L.; Jones, C.T.; Rice, C.M. Architects of assembly: Roles of Flaviviridae non-structural proteins in virion morphogenesis. Nat. Rev. Microbiol. 2008, 6, 699–708.
- Robertson, S.J.; Mitzel, D.N.; Taylor, R.T.; Best, S.M.; Bloom, M.E. Tick-borne flaviviruses: Dissecting host immune responses and virus countermeasures. Immunol. Res. 2009, 43, 172–186.
- Morrison, J.; Aguirre, S.; Fernandez-Sesma, A. Innate immunity evasion by dengue virus. Viruses 2012, 4, 397–413.
- Miorin, L.; Maestre, A.M.; Fernandez-Sesma, A.; Garcia-Sastre, A. Antagonism of type I interferon by flaviviruses. Biochem. Biophys. Res. Commun. 2017, 492, 587–596.
- Gack, M.U.; Diamond, M.S. Innate immune escape by dengue and West Nile viruses. Curr. Opin. Virol. 2016, 20, 119–128.
- Yun, S.I.; Lee, Y.M. Early events in Japanese encephalitis virus infection: Viral entry. Pathogens 2018, 7, 68.
- Hu, T.; Wu, Z.; Wu, S.; Chen, S.; Cheng, A. The key amino acids of E protein involved in early flavivirus infection: Viral entry. Virol. J. 2021, 18, 136.
- Kumar, S.; Verma, A.; Yadav, P.; Dubey, S.K.; Azhar, E.I.; Maitra, S.S.; Dwivedi, V.D. Molecular pathogenesis of Japanese encephalitis and possible therapeutic strategies. Arch. Virol. 2022, 167, 1739–1762.
- Puerta-Guardo, H.; Glasner, D.R.; Espinosa, D.A.; Biering, S.B.; Patana, M.; Ratnasiri, K.; Wang, C.; Beatty, P.R.; Harris, E. Flavivirus NS1 triggers tissue-specific vascular endothelial dysfunction reflecting disease tropism. Cell Rep. 2019, 26, 1598–1613 e1598.
- Rudolph, K.E.; Lessler, J.; Moloney, R.M.; Kmush, B.; Cummings, D.A. Incubation periods of mosquito-borne viral infections: A systematic review. Am. J. Trop. Med. Hyg. 2014, 90, 882–891.
- Solomon, T.; Vaughn, D.W. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr. Top. Microbiol. Immunol. 2002, 267, 171–194.
- Gatus, B.J.; Rose, M.R. Japanese B encephalitis: Epidemiological, clinical and pathological aspects. J. Infect. 1983, 6, 213–218.
- Kuwayama, M.; Ito, M.; Takao, S.; Shimazu, Y.; Fukuda, S.; Miyazaki, K.; Kurane, I.; Takasaki, T. Japanese encephalitis virus in meningitis patients, Japan. Emerg. Infect. Dis. 2005, 11, 471–473.
- Lowry, P.W.; Truong, D.H.; Hinh, L.D.; Ladinsky, J.L.; Karabatsos, N.; Cropp, C.B.; Martin, D.; Gubler, D.J. Japanese encephalitis among hospitalized pediatric and adult patients with acute encephalitis syndrome in Hanoi, Vietnam 1995. Am. J. Trop. Med. Hyg. 1998, 58, 324–329.
- Watt, G.; Jongsakul, K. Acute undifferentiated fever caused by infection with Japanese encephalitis virus. Am. J. Trop. Med. Hyg. 2003, 68, 704–706.
- Xu, Y.; Zhaori, G.; Vene, S.; Shen, K.; Zhou, Y.; Magnius, L.O.; Wahren, B.; Linde, A. Viral etiology of acute childhood encephalitis in Beijing diagnosed by analysis of single samples. Pediatr. Infect. Dis. J. 1996, 15, 1018–1024.
- Poneprasert, B. Japanese encephalitis in children in northern Thailand. Southeast Asian J. Trop. Med. Public Health 1989, 20, 599–603.
- Kumar, R.; Tripathi, P.; Singh, S.; Bannerji, G. Clinical features in children hospitalized during the 2005 epidemic of Japanese encephalitis in Uttar Pradesh, India. Clin. Infect. Dis. 2006, 43, 123–131.
- Kalita, J.; Misra, U.K. Markedly severe dystonia in Japanese encephalitis. Mov. Disord. 2000, 15, 1168–1172.
- Rayamajhi, A.; Singh, R.; Prasad, R.; Khanal, B.; Singhi, S. Clinico-laboratory profile and outcome of Japanese encephalitis in Nepali children. Ann. Trop. Paediatr. 2006, 26, 293–301.
- Solomon, T.; Dung, N.M.; Kneen, R.; Thao le, T.T.; Gainsborough, M.; Nisalak, A.; Day, N.P.; Kirkham, F.J.; Vaughn, D.W.; Smith, S.; et al. Seizures and raised intracranial pressure in Vietnamese patients with Japanese encephalitis. Brain 2002, 125, 1084–1093.
- Misra, U.K.; Kalita, J. Seizures in Japanese encephalitis. J. Neurol. Sci. 2001, 190, 57–60.
- Solomon, T.; Dung, N.M.; Kneen, R.; Gainsborough, M.; Vaughn, D.W.; Khanh, V.T. Japanese encephalitis. J. Neurol. Neurosurg. Psychiatry 2000, 68, 405–415.
- Lagarde, S.; Lagier, J.C.; Charrel, R.; Querat, G.; Vanhomwegen, J.; Despres, P.; Pelletier, J.; Kaphan, E. Japanese encephalitis in a French traveler to Nepal. J. Neurovirol. 2014, 20, 99–102.
- Kakoti, G.; Dutta, P.; Ram Das, B.; Borah, J.; Mahanta, J. Clinical profile and outcome of Japanese encephalitis in children admitted with acute encephalitis syndrome. Biomed. Res. Int. 2013, 2013, 152656.
- Borah, J.; Dutta, P.; Khan, S.A.; Mahanta, J. A comparison of clinical features of Japanese encephalitis virus infection in the adult and pediatric age group with acute encephalitis syndrome. J. Clin. Virol. 2011, 52, 45–49.
- Misra, U.K.; Kalita, J. Movement disorders in Japanese encephalitis. J. Neurol. 1997, 244, 299–303.
- Misra, U.K.; Kalita, J. Prognosis of Japanese encephalitis patients with dystonia compared to those with parkinsonian features only. Postgrad. Med. J. 2002, 78, 238–241.
- Misra, U.K.; Kalita, J. Anterior horn cells are also involved in Japanese encephalitis. Acta Neurol. Scand. 1997, 96, 114–117.
- Solomon, T.; Kneen, R.; Dung, N.M.; Khanh, V.C.; Thuy, T.T.; Ha, D.Q.; Day, N.P.; Nisalak, A.; Vaughn, D.W.; White, N.J. Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet 1998, 351, 1094–1097.
- Kumar, S.; Misra, U.K.; Kalita, J.; Salwani, V.; Gupta, R.K.; Gujral, R. MRI in Japanese encephalitis. Neuroradiology 1997, 39, 180–184.
- Shen, Q.; Li, Y.; Lu, H.; Ning, P.; Huang, H.; Zhao, Q.; Xu, Y. Acute flaccid paralysis as the initial manifestation of Japanese encephalitis: A case report. Jpn. J. Infect. Dis. 2020, 73, 381–382.
- Kalita, J.; Misra, U.K. Comparison of CT scan and MRI findings in the diagnosis of Japanese encephalitis. J. Neurol. Sci. 2000, 174, 3–8.
- Chung, C.C.; Lee, S.S.; Chen, Y.S.; Tsai, H.C.; Wann, S.R.; Kao, C.H.; Liu, Y.C. Acute flaccid paralysis as an unusual presenting symptom of Japanese encephalitis: A case report and review of the literature. Infection 2007, 35, 30–32.
- Basumatary, L.J.; Raja, D.; Bhuyan, D.; Das, M.; Goswami, M.; Kayal, A.K. Clinical and radiological spectrum of Japanese encephalitis. J. Neurol. Sci. 2013, 325, 15–21.
- Ma, J.; Zhang, T.; Jiang, L. Japanese encephalitis can trigger anti-N-methyl-D-aspartate receptor encephalitis. J. Neurol. 2017, 264, 1127–1131.
- Ma, J.; Han, W.; Jiang, L. Japanese encephalitis-induced anti-N-methyl-d-aspartate receptor encephalitis: A hospital-based prospective study. Brain Dev. 2020, 42, 179–184.
- Tian, M.; Li, J.; Lei, W.; Shu, X. Japanese encephalitis virus-induced anti-N-methyl-D-aspartate receptor encephalitis: A case report and review of literature. Neuropediatrics 2019, 50, 111–115.
- Verma, R.; Praharaj, H.N.; Patil, T.B.; Giri, P. Acute transverse myelitis following Japanese encephalitis viral infection: An uncommon complication of a common disease. BMJ Case Rep. 2012, 2012, bcr2012007094.
- Ankur Nandan, V.; Nilesh, K.; Dibyaranjan, B.; Ashutosh, T.; Ravi, A.; Arvind, A. Acute transverse myelitis (ascending myelitis) as the initial manifestation of Japanese encephalitis: A rare presentation. Case Rep. Infect. Dis. 2013, 2013, 487659.
- Chen, W.L.; Liao, M.F.; Chiang, H.L.; Lin, S.K. A possible case of acute disseminated encephalomyelitis after Japanese encephalitis. Acta Neurol. Taiwan. 2013, 22, 169–173.
- Ohtaki, E.; Matsuishi, T.; Hirano, Y.; Maekawa, K. Acute disseminated encephalomyelitis after treatment with Japanese B encephalitis vaccine (Nakayama-Yoken and Beijing strains). J. Neurol. Neurosurg. Psychiatry 1995, 59, 316–317.
- Lincoln, A.F.; Sivertson, S.E. Acute phase of Japanese B encephalitis; two hundred and one cases in American soldiers, Korea, 1950. J. Am. Med. Assoc. 1952, 150, 268–273.
- Sarkari, N.B.; Thacker, A.K.; Barthwal, S.P.; Mishra, V.K.; Prapann, S.; Srivastava, D.; Sarkari, M. Japanese encephalitis (JE) part I: Clinical profile of 1282 adult acute cases of four epidemics. J. Neurol. 2012, 259, 47–57.
- Burke, D.S.; Nisalak, A.; Ussery, M.A.; Laorakpongse, T.; Chantavibul, S. Kinetics of IgM and IgG responses to Japanese encephalitis virus in human serum and cerebrospinal fluid. J. Infect. Dis. 1985, 151, 1093–1099.
- Diagana, M.; Preux, P.M.; Dumas, M. Japanese encephalitis revisited. J. Neurol. Sci. 2007, 262, 165–170.
- Ma, J.; Jiang, L. Outcome of children with Japanese encephalitis and predictors of outcome in southwestern China. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 660–665.
- Lo, S.H.; Tang, H.J.; Lee, S.S.; Lee, J.C.; Liu, J.W.; Ko, W.C.; Chang, K.; Lee, C.Y.; Chang, Y.T.; Lu, P.L. Determining the clinical characteristics and prognostic factors for the outcomes of Japanese encephalitis in adults: A multicenter study from southern Taiwan. J. Microbiol. Immunol. Infect. 2019, 52, 893–901.
- Huy, B.V.; Tu, H.C.; Luan, T.V.; Lindqvist, R. Early mental and neurological sequelae after Japanese B encephalitis. Southeast Asian J. Trop. Med. Public Health 1994, 25, 549–553.
- Schneider, R.J.; Firestone, M.H.; Edelman, R.; Chieowanich, P.; Pornpibul, R. Clinical sequelae after Japanese encephalitis: A one year follow-up study in Thailand. Southeast Asian J. Trop. Med. Public Health 1974, 5, 560–568.
- Mayxay, M.; Douangdala, P.; Vilayhong, C.; Phommasone, K.; Chansamouth, V.; Vongsouvath, M.; Rattanavong, S.; Chang, K.; Sengvilaipaseuth, O.; Chanthongthip, A.; et al. Outcome of Japanese encephalitis virus (JEV) infection in pediatric and adult patients at Mahosot Hospital, Vientiane, Lao PDR. Am. J. Trop. Med. Hyg. 2021, 104, 567–575.
- Sarkari, N.B.; Thacker, A.K.; Barthwal, S.P.; Mishra, V.K.; Prapann, S.; Srivastava, D.; Sarkari, M. Japanese encephalitis (JE) part II: 14 years’ follow-up of survivors. J. Neurol. 2012, 259, 58–69.
- Turtle, L.; Easton, A.; Defres, S.; Ellul, M.; Bovill, B.; Hoyle, J.; Jung, A.; Lewthwaite, P.; Solomon, T. ‘More than devastating’-patient experiences and neurological sequelae of Japanese encephalitis section sign. J. Travel Med. 2019, 26, taz064.
- Xiang, J.Y.; Zhang, Y.H.; Tan, Z.R.; Huang, J.; Zhao, Y.W. Guillain-Barre syndrome associated with Japanese encephalitis virus infection in China. Viral Immunol. 2014, 27, 418–420.
- Wang, G.; Li, H.; Yang, X.; Guo, T.; Wang, L.; Zhao, Z.; Sun, H.; Hou, X.; Ding, X.; Dou, C.; et al. Guillain-Barre syndrome associated with JEV infection. N. Engl. J. Med. 2020, 383, 1188–1190.
- Ravi, V.; Taly, A.B.; Shankar, S.K.; Shenoy, P.K.; Desai, A.; Nagaraja, D.; Gourie-Devi, M.; Chandramuki, A. Association of Japanese encephalitis virus infection with Guillain-Barre syndrome in endemic areas of south India. Acta Neurol. Scand. 1994, 90, 67–72.
- Esposito, S.; Longo, M.R. Guillain-Barre syndrome. Autoimmun. Rev. 2017, 16, 96–101.
- Joseph, N.; Piccione, E.A. Guillain-Barre syndrome triggered by West Nile virus: A rare case scenario. J. Clin. Neuromuscul. Dis. 2019, 21, 54–56.
- Ahmed, S.; Libman, R.; Wesson, K.; Ahmed, F.; Einberg, K. Guillain-Barre syndrome: An unusual presentation of West Nile virus infection. Neurology 2000, 55, 144–146.
- Krauer, F.; Riesen, M.; Reveiz, L.; Oladapo, O.T.; Martinez-Vega, R.; Porgo, T.V.; Haefliger, A.; Broutet, N.J.; Low, N.; WHO Zika Causality Working Group. Zika virus infection as a cause of congenital brain abnormalities and Guillain-Barre syndrome: Systematic review. PLoS Med. 2017, 14, e1002203.
- Nascimento, O.J.M.; da Silva, I.R.F. Guillain-Barre syndrome and Zika virus outbreaks. Curr. Opin. Neurol. 2017, 30, 500–507.
- Carod-Artal, F.J.; Wichmann, O.; Farrar, J.; Gascon, J. Neurological complications of dengue virus infection. Lancet Neurol. 2013, 12, 906–919.
- Li, G.H.; Ning, Z.J.; Liu, Y.M.; Li, X.H. Neurological manifestations of dengue infection. Front. Cell. Infect. Microbiol. 2017, 7, 449.
- Benavides-Lara, A.; la Paz Barboza-Arguello, M.; Gonzalez-Elizondo, M.; Hernandez-deMezerville, M.; Brenes-Chacon, H.; Ramirez-Rojas, M.; Ramirez-Hernandez, C.; Arjona-Ortegon, N.; Godfred-Cato, S.; Valencia, D.; et al. Zika virus-associated birth defects, Costa Rica, 2016–2018. Emerg. Infect. Dis. 2021, 27, 360–371.
- Chaturvedi, U.C.; Mathur, A.; Chandra, A.; Das, S.K.; Tandon, H.O.; Singh, U.K. Transplacental infection with Japanese encephalitis virus. J. Infect. Dis. 1980, 141, 712–715.
- Patgiri, S.J.; Borthakur, A.K.; Borkakoty, B.; Saikia, L.; Dutta, R.; Phukan, S.K. An appraisal of clinicopathological parameters in Japanese encephalitis and changing epidemiological trends in upper Assam, India. Indian J. Pathol. Microbiol. 2014, 57, 400–406.
- Mathur, A.; Tandon, H.O.; Mathur, K.R.; Sarkari, N.B.; Singh, U.K.; Chaturvedi, U.C. Japanese encephalitis virus infection during pregnancy. Indian J. Med. Res. 1985, 81, 9–12.
- Mathur, A.; Chaturvedi, U.C.; Tandon, H.O.; Agarwal, A.K.; Mathur, G.P.; Nag, D.; Prasad, A.; Mittal, V.P. Japanese encephalitis epidemic in Uttar Pradesh, India during 1978. Indian J. Med. Res. 1982, 75, 161–169.
- Shimizu, T.; Kawakami, Y.; Fukuhara, S.; Matumoto, M. Experimental stillbirth in pregnant swine infected with Japanese encephalitis virus. Jpn. J. Exp. Med. 1954, 24, 363–375.
- Burns, K.F. Congenital Japanese B encephalitis infection of swine. Proc. Soc. Exp. Biol. Med. 1950, 75, 621–625.
- Hosoya, H.; Matumoto, M.; Iwasa, S. Epizootiological studies on stillbirth of swine occurred in Japan during summer months of 1948. Jpn. J. Exp. Med. 1950, 20, 587–595.
- Tsubaki, S.; Masu, S.; Obata, Y.; Shimada, F. Studies on Japanese B encephalitis on swine encephalitis and abortion (1947–1949). Kitasato Arch. Exp. Med. 1950, 23, 9–12.
- Desingu, P.A.; Ray, P.K.; Patel, B.H.; Singh, R.; Singh, R.K.; Saikumar, G. Pathogenic and genotypic characterization of a Japanese encephalitis virus isolate associated with reproductive failure in an Indian pig herd. PLoS ONE 2016, 11, e0147611.
This entry is offline, you can click here to edit this entry!
