Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
As fruit-derived foods are perishable, their processing plays a crucial role in guaranteeing their safety and extending their shelf life. Fruit preservation techniques are based mainly on the use of heat treatments or synthetic preservatives. The use of natural antimicrobials in the food industry is being proposed as an eco-friendly postharvest technology to preserve fruit-derived foods.
- naturally occurring antimicrobials
- covalent immobilization
- food preservatives
- processing aids
- juice
- jam
- wine
- soft drink
1. Using Natural Antimicrobials in Fruit-Derived Foods
The systematic review on the use of natural antimicrobials in fruit-derived foods revealed that essential oil components (EOCs), bacteriocins, polysaccharides, organic acids, or bacteriophages are the most frequent molecules employed as preservatives in fruit-derived foods, probably due to their consideration as GRAS (generally recognized as safe) products by the U.S. FDA [1].
EOCs are a mixture of different compounds, such as terpenes, alcohols, phenols, etc., generated from plants. Free hydroxyl functional groups (-OH) are mostly responsible for their antimicrobial activity [2]. Bacteriocins are the peptides obtained from bacteria that are capable of changing the permeability of microorganisms by provoking their lysis. Of all the bacteriocins, nisin, iturin A, natamycin, bovicin, and thurincin H are proposed as antimicrobials [3][4][5][6][7]. The most important polysaccharide is chitosan, a biopolymer generated by the deacetylation processes of chitin. Its antimicrobial action is based mainly on the interaction between chitosan cationic groups and microorganisms [8]. Organic acids cover different compounds that present a deprotonated carboxyl group at a neutral pH. The interaction between the active group and a microorganism’s membrane is the main antimicrobial mechanism [9]. Bacteriophages are viruses that penetrate a specific bacterial host, spread within the host, and release more phages after cell lysis [10].
The antimicrobial activity of the naturally occurring compounds was tested in different fruit-derived products (i.e., wine, fruit juices, or soft drinks) against bacteria, such as Escherichia coli, Salmonella enterica, and Listeria monocytogenes, yeasts, such as Zygosaccharomyces bailii, Zygosaccharomyces rouxii, and Saccharomyces cerevisiae. In the reviewed studies, the authors reported remarkable efficacy for the microbiological control of all the fruit-derived foods. Table 1 summarizes the different studies that evaluated the antimicrobial efficacy of the aforementioned compounds in fruit-derived foods.
Despite the demonstrated in vivo inhibitory efficacy of antimicrobials, some studies have revealed that their direct addition to food presents certain limitations that stem from their intrinsic physico-chemical properties. Mitropoulou et al. (2020) [11] and Thomas-Popo et al. (2019) [2] expressed that essential oils (EOs) and their components (EOCs) are poorly soluble in aqueous media and highly volatile when studied for antimicrobial purposes in fruit juices. Campion et al. (2017) [12] indicated that nisin is more stable at an acid pH when tested in milk and apple juice media. Liao et al. (2017) [4] reported that the temperature and salt concentration of media can affect nisin activity for apple juice stabilization.
In addition to all these limitations, some reports have shown that the incorporation of these compounds modifies some food sensory attributes. The study of Beristaín-Bauza et al. (2018) [13] evaluated the sensory impact of 0–100 µg/mL of cinnamaldehyde and vanillin in coconut water. These authors reported the lowest general acceptability values at the highest concentration for both antimicrobial compounds. Chung et al. (2018) [14] tested the addition of thymol to different citrus extracts (lime, lemon, and calamarsi) and quantified a lower overall acceptability (p < 0.05) by incorporating thymol at concentrations up to 2 mM. Further research rated the appearance, odor, taste, aftertaste, viscosity, and overall acceptance parameters at different concentrations of isoeugenol (0–1 µL/mL) added to pineapple juice [2]. The results showed a significant decrease (p < 0.05) in the odor, taste, aftertaste, and overall acceptance scores, while no changes were recorded for the appearance and viscosity characteristics. Using EOs from Citrus medica and Cinnamomum zeylanicum for microbiological wine stabilization [11], aroma and taste assays were carried out. The results indicated that the incorporation of EOs significantly affected both parameters. Indeed, the product was rejected when concentrations over 0.010% of EOs were added because EOs masked the wine taste and also formed an oily layer on wine.
The interaction of antimicrobial compounds with food matrix components also limits their application in food products [2][6][10][15][16][17][18][19]. Certain nutrients in food can have a protective effect on microorganisms; therefore, it would be necessary to use higher concentrations of natural antimicrobials. However, increasing the antimicrobial effective dose can result in more limitations that derive from applying a high concentration of certain natural antimicrobial compounds, as previously highlighted.
For all these reasons, research is currently exploring new alternative dosage forms of natural antimicrobials to be used as food preservatives or processing aids, such as encapsulation of immobilization. The first alternative consists of trapping an active agent (i.e., a natural antimicrobial) in a carrier material to enhance its later release in the food or in the gastrointestinal tract. Immobilization, in contrast, consists of anchoring the active biomolecule on the surface of the support. This technology not only makes it possible to preserve the native antimicrobial properties of the active biomolecule but also prevents its leaching into the food matrix due to the creation of covalent bonds between the support and the antimicrobial compound.
Having this in mind, covalent immobilization is presented in this research as an eco-friendly postharvest technology with great possibilities for application in the preservation of fruit-derived foods.
Table 1. Relevant studies by applying free natural antimicrobial compounds in fruit-derived foods.
| Antimicrobial | Food Matrix | Target Microorganisms | Effect on Food Product | Ref. |
|---|---|---|---|---|
| Essential oils or their components | ||||
| Combination of carvacrol and nisin | Apple juice | Escherichia coli O157:H7 | The nisin and carvacrol combination caused the complete inhibition of the bacterium after 3 h of incubation at room temperature. | [12] |
| Oregano and thyme EOs | Wine | E. coli, Salmonella enterica | Both EOs were more active against S. enterica than E. coli when added to wine. | [20] |
| Vanillin and cinnamaldehyde | Coconut water | S. enterica serovar Typhimurium | The complete elimination of the bacterium was achieved when adding 100 µg/mL of cinnamaldehyde, while the addition of vanillin only delayed microorganism growth. Both antimicrobials produced undesirable sensory characteristics at 100 µg/mL. | [13] |
| Thymol | Calamansi juice | E. coli 0157:H7, S. enterica serovar Typhimurium | The use of thymol (20 mM) led to the total elimination of all the tested microorganisms. Nevertheless, thymol incorporation negatively affected the sensory profile of the food-derived product. | [14] |
| Cinnamon leaf EO | Orange juice | Saccharomyces cerevisiae | Cinnamon EO (650 mg/mL) brought about a reduction in yeast of ca. 4 log CFU/mL. | [21] |
| Thymbra capitata EO | Pomegranate juice | Aerobic mesophilic bacteria, Streptococcus thermophilus, yeasts and molds | For all the microorganisms, the addition of the EO at 0.125% (v/v) provoked a significant reduction (4 log CFU/mL) when incubated at 4 and 25 °C, while no impact on physico-chemical characteristics was detected. | [15] |
| Mint EO, carvacrol, and natamycin | Apple juice | Zygosaccharomyces bailii, Zygosaccharomyces rouxii | The employed antimicrobial preservatives were able to reduce ethanol formation owing to microorganisms inhibition. | [3] |
| Isoeugenol | Pineapple juice | E. coli O157:H7, S. enterica, Listeria monocytogenes | The addition of 0.5 µL/mL of the EOC managed to reduce >5 log CFU/mL in all the tested microorganisms. The antimicrobial modified some sensory parameters. | [2] |
| Eucalyptus globulus EO (EGEO) | Orangina fruit juice | S. cerevisiae | EGEO in combination with heat treatment (70 °C for 2 min) at concentrations ranging from 0.8 to 4 µL/mL was effective in reducing S. cerevisiae growth in juice. | [16] |
| Citrus medica and Cinnamomum zeylanicum EOs | Wine | Oenococcus oeni, Pediococcus pentosaceus, Gluconobacter cerinus, Brettanomyces bruxellensis, Candida zemplinina, Hanseniaspora uvarum, Pichia guilliermondii, Z. bailii | Both extracts extended the shelf life from 9 days (when not applying EOs) to 18 and 74 days when using C. medica and C. zeylanicum EOs, respectively. The addition of EOs modified the sensory profile. The product was rejected when the EO concentration was raised to up to 0.010%. | [11] |
| Citrus lemon and Citrus reticulata EOs | Orange juice | Lactobacillus brevis, Leuconostoc mesenteroides | The use of both EOs alone (0.5 μL/mL) or combined caused bacterial reduction. | [22] |
| Carvacrol, thymol, and trans-cinnamaldehyde | Apple juice | Z. rouxii | All the EOCs provoked fungi reduction of ca. 100% when applied to apple juice. | [23] |
| Vanillin | Apple juice | S. enterica serovar Typhimurium | The microorganism was inactivated after 75 min at 45 °C using 3.2 mg/mL of vanillin. | [24] |
| Cinnamon bark and thyme EOs, and thymol | Tomato juice | L. monocytogenes | The combined use of EOCs at 0.6250 μL/mL showed the best antimicrobial results. The bacterium was totally eliminated (>5.2 log CFU/mL) after 24 h of incubation at both 25 °C and 10 °C in tomato juice. | [25] |
| Pistacia lentiscus and Fortunella margarita EOs | Fruit juice (lemon, apple, and blackcurrant) | Aspergillus niger, S. cerevisiae | The combination of EOs (P. lentiscus EO 0.2% (w/w) and F. margarita EO 0.006% (w/w) reduced fungi growth to <100 spores/mL, while treatment was not effective against S. cerevisiae. Higher concentrations of EOs than those tested were rejected because they produced an intense bitter taste. | [18] |
| Thymol and trans cinnamaldehyde | Apple juice | Z. rouxii | The use of 0.125 and 1.25 of thymol and trans cinnamaldehyde mg/mL totally reduced in apple juice. | [26] |
| Melissa officinalis EO (MEEO) | Watermelon juice | L. monocytogenes | The use of 2 µL/mL of MEEO resulted in a complete bacterial growth reduction from day 2 to day 7 in inoculated watermelon juice. | [1] |
| Bacteriocins | ||||
| Nisin | Apple juice | Aerobic bacteria, molds, and yeasts | The addition of nisin lowered the aerobic bacteria counts, while no changes appeared for molds and yeasts. The treatment with the antimicrobial did not change the product’s color. | [4] |
| Iturin A | Orange juice | S. cerevisiae | The addition of iturin A (0.76 mg/mL) completely inhibited the target microorganism after 4 days of incubation. | [7] |
| Lactobacillus plantarum Cys5-4 | Orange juice | E. coli, S. enterica | The application of the bacterium (128 AU/mL) reduced the E. coli population ca. 3 log CFU/mL after 5 days of incubation, while no antimicrobial effects were shown for S. enterica. | [19] |
| Nisin | Coconut water | Yeasts, molds, and total coliforms | The use of nisin (50 ppm) reduced the counts of both yeasts and molds (below 1 log CFU/mL), while the total coliforms were not detected. The sensory profile did not change after adding nisin. | [27] |
| Nisin | Apple juice | E. coli, Listeria innocua | Treatment with nisin (500 IU/mL) reduced 1.5 and 3 log CFU/mL for E. coli and L. innocua, respectively, after 30 min of incubation at 37 °C. The pH of the product was lower (p < 0.05) because nisin is more stable at an acid pH. | [28] |
| Nisin | Orange Juice | Total aerobic bacteria | The combination of thermosonication and nisin treatment led to remarkable bacterial reduction. The use of nisin increased the product’s sensory acceptability. | [29] |
| Kenaf seed peptides | Mango and pineapple juices | S. enterica serovar Typhimurium, E. coli, L. monocytogenes | The antimicrobial compound (3000 mg/mL) reduced all the tested bacteria (>5 log CFU/mL) for 150 days in both media, while no color changes were detected. | [30] |
| Bovicin HC5 and nisin | Pineapple, orange, papaya, grape, mango, and apple juices | Alicyclobacillus acidoterrestris | Treatment with 80 AU/mL of bovicin or nisin totally eliminated the bacterium vegetative cells and the thermal resistance of their endospores. | [5] |
| Thurincin H | Orange juice | E. coli, L. innocua | An amount of 40 μg/mL of the antimicrobial compound reduced L. innocua by 5.5 log CFU/mL but only 1 log CFU/mL of E. coli. | [6] |
| Lactobacillus acidophilus NX2-6 | Apple juice | A. acidoterrestris | The bacterium growth was inhibited by adding 0.2% of supernatant containing acidocin NX2-6 at 28 °C. The addition of the antimicrobial increased the quality of the product. Concretely, it was enlarged the storage time in a transparent and precipitation-free state. | [31] |
| Pediococcus acidilactici NCDC 252 | Apple juice, apricot pulp, and pre-pasteurized wine | E. coli | The use of 1 mg/mL of the antimicrobial reduced the E. coli population by ca. 3 log CFU/mL. | [17] |
| Polysaccharides | ||||
| Chitosan | Wine | Acetobacter malorum and Acetobacter pasteurianus | The use of chitosan totally inhibited A. pasteurianus, while the same treatment reduced the A. malorum population by 50% after 15 days of inoculation. | [32] |
| Chitosan | Wine | B. bruxellensis | Antimicrobial activity depended on the strain of the used microorganism. Indeed, 41% of the assayed strain were reduced by chitosan. | [33] |
| Chitosan | Wine | S. cerevisiae, acetic acid bacteria strains, lactic acid bacteria strains, and O. oeni | The use of chitosan was effective for lactic acid bacteria and O. oeni but acetic acid bacteria and S. cerevisiae were barely reduced. | [8] |
| Organic acids | ||||
| Gallic acid and ferulic acid | Apple juice | E. coli, L. innocua | The application of gallic acid (10 mM) and ferulic acid (1 mM) was able to reduce both microorganisms from 6 log CFU/mL to below the detection limit. | [34] |
| β-resorcylic acid and caprylic acid | Orange juice | S. enterica serovar Typhimurium | The combination of both antimicrobial compounds (8.43 mM of β-resorcylic acid and 0.10 mM of caprylic acid) lowered the temperature needed to reach the microbial parameters corresponding to pasteurization. Color and flavor did not change after treatment. | [35] |
| p-Coumaric acid | Apple juice | A. acidoterrestris | The addition of the antimicrobial compound (0.4 mg/mL) accelerated the degradation of vegetative cells from 5 to 3 days at 4 °C. The study did not show any changes in the aroma profile after treatment. | [36] |
| Bacteriophages | ||||
| Lytic phage vB_SalS-LPSTLL | Apple juice | S. enterica serovar Typhimurium | The addition of the bacteriophage reduced (p < 0.05) bacteria counts by up to 0.5 log CFU/mL in apple juice. Treatment did not change the sensory quality of the fruit-derived product. | [10] |
2. Using Immobilized Antimicrobials in Fruit-Derived Foods
The use of immobilized natural antimicrobials to preserve or extend the shelf life of fruit-derived products has increased in recent years owing to their marked antimicrobial effectiveness and good capacity to cushion their sensory and stability impact on foods after grafting [37][38][39].
Immobilization refers to the chemical, physico-chemical, or electrostatic binding of bioactive molecules to a surface. Chemical immobilization involves the formation of at least one covalent bond between the surface and the target biomolecule, which represents the most permanent and irreversible form of coupling. Covalent linkage involves strongly bounding the compound of interest with a potentially longer shelf life, greater bioactivity, and lower toxicological risk [40]. The immobilization of natural antimicrobials on the surface of different materials is an approach that allows contact-killing materials to be obtained through antimicrobial molecules that are covalently attached to the surface. With this immobilization procedure, antimicrobials are exposed to the external environment, which enables direct contact between the immobilized molecule and the target microorganism [41].
According to this research, these antimicrobial systems can be applied to fruit-derived foods in different processing stages: (i) as a food additive (preservative) present in the final product and (ii) as food processing aids that are absent in the final product (see Figure 1). This section focuses on discussing application cases of immobilized natural antimicrobials in fruit-derived products by differentiating these two application approaches.
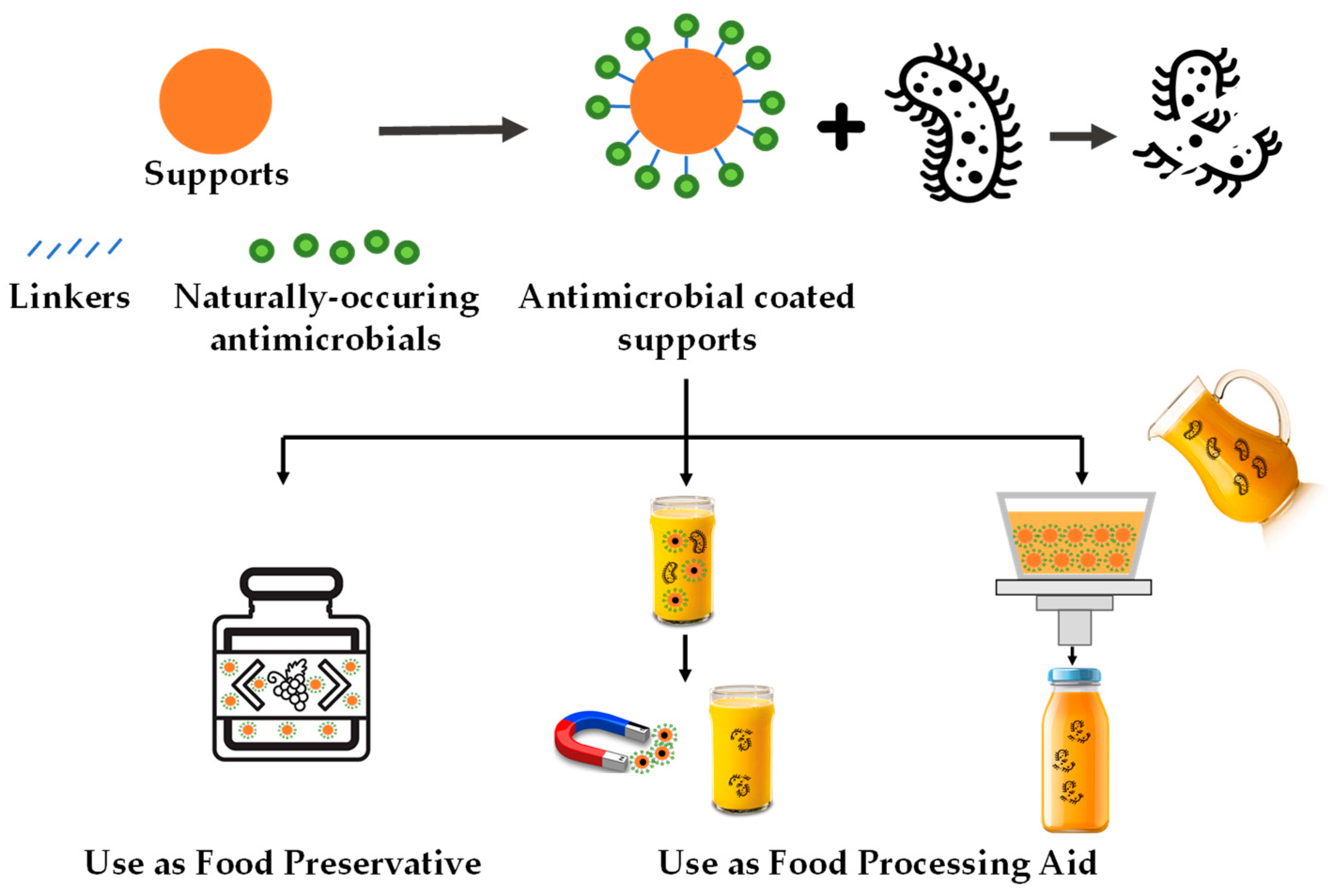
Figure 1. Schematic representation of the main uses of natural antimicrobial compounds immobilized on food-grade supports for fruit-derived food preservation.
2.1. Use as Food Preservatives
In the last few years, the design of immobilized antimicrobial systems as food preservatives to control or prevent microbial spoilage in fruit-derived foods has grown.
In this context, Ribes et al. (2017) [39] conducted the first work, in which EOCs immobilized by an imine bond on the surface of silica supports were employed as promising antifungal agents to control strawberry jam decay without altering the final product’s sensory perception. Based on the marked antimicrobial activity of these promising preservatives, Ribes et al. (2019) [42] also investigated the synergistic effect of EOCs immobilized on the surface of silica particles against the bacteria and yeasts present in fruit juice and their influence on the food matrix. This research demonstrated the feasibility of combining immobilized antimicrobials to improve the microbial stabilization of fruit juice given that immobilization masks the undesirable aroma of EOCs in the food matrix according to both the gas chromatography and sensory evaluation results. Similarly, the antimicrobial activity of thymol immobilized on hollow mesoporous silica particles (HMSNs) in a real food system was investigated for the first time by Liu et al. (2022) [43]. It is noteworthy in this research that the antimicrobial agent was covalently grafted to the silica support by running a reaction with 3-(triethoxysilyl)-propyl isocyanate, which resulted in carbamate bonding instead of the imine bonding described in the above-mentioned examples. The EOC immobilized on HMSNs showed an excellent potential for enhancing the antimicrobial activity of thymol against foodborne bacteria. It also reduced the impact of EOCs on the final product’s physico-chemical properties, such as color, pH, and soluble solids content. However, the impacts on the most relevant organoleptic properties (aroma and flavor) were not evaluated. Recently, different antimicrobial systems based on the covalent immobilization of chitosan for the microbial control of apple juice were developed by Ruiz-Rico et al. (2023) [44]. The use of chitosan-coated supports as food preservatives in juice reduced the food matrix’s microbial load, which increased its shelf life, although the impact on juice was not evaluated. All these examples confirm the potential of antimicrobial systems based on immobilized natural antimicrobials as food preservatives in the food industry after regulatory authorities have approved them.
Finally, it is worth mentioning that all these reviewed systems present common characteristics: (i) the bioactive compound is covalently immobilized on the surface of the support; (ii) immobilized bioactive compounds exhibit greater antimicrobial or antifungal activity than their free form; (iii) the use of these novel preservatives does not modify the final product’s physico-chemical or sensory properties.
2.2. Use as Food Processing Aid
The second approach of using immobilized natural antimicrobials in fruit-derived foods does not imply the permanence of particles in food to exert their antimicrobial effect. So in this case, instead of considering the antimicrobial supports to be food preservatives, they should be taken as processing aids.
In the systematic review of this application, four works used immobilized antimicrobials as processing aids. Within this framework, Song et al. (2019) [45] employed iron oxide nanoparticle–polydopamine–nisin composites with magnetic characteristics to control A. acidoterrestris growth in apple juice and recovered particles after treatment. After demonstrating that the juices treated with functionalized magnetic particles were not influenced in physico-chemical and sensory terms, as well as the non-toxicity and biosecurity of composites, the authors highlighted this innovative antimicrobial system as a promising tool to control A. acidoterrestris contamination in the juice industry.
Conversely, the other three studies applied the immobilization of natural antimicrobial compounds on food-grade supports as a strategy to create filtration systems for the cold pasteurization of liquid fruit-derived foods. The main objective of these filtration systems was to remove spoilage or pathogenic microorganisms from liquid fruit-derived products (juices or wine) through the retention and/or the disruption of the bacterial cell wall after the interaction with the antimicrobial compound [46]. Zhang et al. (2021) [47] reported employing nisin-coated polyvinylidene difluoride microfiltration membranes (pore diameter of 0.22 μm) to eliminate A. acidoterrestris contamination from apple juice due to the antibacterial action of nisin and the retention of spores on the membrane surface. In a different approach, Peña-Gomez et al. (2019) [38] developed novel filtering materials based on silica microparticles (50 μm) functionalized with EOCs as an alternative cold pasteurization method for apple juice by depth filtration. In a first assay, the developed filtration system was able to reduce the E. coli load inoculated in pasteurized apple juice of at least 5 logarithmic reduction values (LRVs). In addition, employing antimicrobial particles for the filtration of fresh juices was able to microbiologically stabilize the non-thermally treated apple juice, which resulted in juice with high microbial stability and quality. This suggests that this filtration technology is a promising alternative to existing pasteurization technologies that apply heat. In another work, Ruiz-Rico et al. (2021) [48] evaluated filter aids based on the covalent immobilization of different EOCs and other phenolic compounds on wine microbiological stabilization. These filtering aids presented some advantages over standard filtration materials, such as minimal impact on wine sensory characteristics and high removal capacity. Likewise, they could be used for clarification, microbiological stabilization, and sterile filtration in a single continuous treatment, thus reducing wine losses and energy costs by compiling different traditional filtration stages in a single step, as well as enhancing the treatment’s overall hygiene and security. However, the authors pointed out the need to improve the stability of grafting and the reuse conditions or filter life before being applied in the food industry.
These described filtration systems differ from micro- and ultrafiltration techniques, which have been extensively studied and implemented for the cold pasteurization of drinks on an industrial scale, in terms of removal capacity and their impact on the properties of filtered drinks. The mechanism of action of micro- and ultrafiltration (pore size from 0.001 μm to <0.1 μm for ultrafiltration, and from 0.1 μm to 10 μm for microfiltration) is the physical retention of all the molecules and organisms bigger than the pore size. In contrast, filtration systems based on membranes or particles functionalized with natural antimicrobials exhibit a larger pore size or filtration channels that only allow the partial retention of microorganisms and food matrix components. Therefore, they act by the combined effect of cell retention and cell damage due to the specific interaction with the antimicrobial compound by preserving the nutritional, functional, and sensory properties of the filtered drink.
This entry is adapted from the peer-reviewed paper 10.3390/foods12102060
References
- Carvalho, F.; Coimbra, A.T.; Silva, L.; Duarte, A.P.; Ferreira, S. Melissa Officinalis Essential Oil as an Antimicrobial Agent against Listeria Monocytogenes in Watermelon Juice. Food Microbiol. 2023, 109, 104105.
- Thomas-Popo, E.; Mendonca, A.; Dickson, J.; Shaw, A.; Coleman, S.; Daraba, A.; Jackson-Davis, A.; Woods, F. Isoeugenol Significantly Inactivates Escherichia Coli O157:H7, Salmonella Enterica, and Listeria Monocytogenes in Refrigerated Tyndallized Pineapple Juice with Added Yucca Schidigera Extract. Food Control 2019, 106, 106727.
- Karaman, K.; Sagdic, O. Zygosaccharomyces Bailii and Z. Rouxii Induced Ethanol Formation in Apple Juice Supplemented with Different Natural Preservatives: A Response Surface Methodology Approach. J. Microbiol. Methods 2019, 163, 105659.
- Liao, H.; Jiang, L.; Cheng, Y.; Liao, X.; Zhang, R. Application of Nisin-Assisted Thermosonication Processing for Preservation and Quality Retention of Fresh Apple Juice. Ultrason. Sonochem. 2017, 42, 244–249.
- Ribeiro, A.M.; Paiva, A.D.; Cruz, A.M.; Vanetti, M.C.; Ferreira, S.O.; Mantovani, H.C. Bovicin HC5 and Nisin Reduce Cell Viability and the Thermal Resistance of Alicyclobacillus Acidoterrestris Endospores in Fruit Juices. J. Sci. Food Agric. 2022, 102, 3994–4002.
- Ruiz-De Anda, D.; Casados-Vazquez, L.E.; Ozuna, C. The Synergistic Effect of Thurincin H and Power Ultrasound: An Alternative for the Inactivation of Listeria Innocua ATCC 33090 and Escherichia Coli K-12 in Liquid Food Matrices. Food Control 2022, 135, 108778.
- Shi, J.; Zhu, X.; Lu, Y.; Zhao, H.; Lu, F.; Lu, Z. Improving Iturin A Production of Bacillus Amyloliquefaciens by Genome Shuffling and Its Inhibition against Saccharomyces Cerevisiae in Orange Juice. Front. Microbiol. 2018, 9, 2683.
- Miot-Sertier, C.; Paulin, M.; Dutilh, L.; Ballestra, P.; Albertin, W.; Masneuf-Pomarede, I.; Coulon, J.; Moine, V.; Vallet-Courbin, A.; Maupeu, J.; et al. Assessment of Chitosan Antimicrobial Effect on Wine Microbes. Int. J. Food Microbiol. 2022, 381, 109907.
- Wang, Q.; Falcao De Oliveira, E.; Alborzi, S.; Bastarrachea, L.J.; Tikekar, R.V. On Mechanism behind UV-A Light Enhanced Antibacterial Activity of Gallic Acid and Propyl Gallate against Escherichia Coli O157:H7. Sci. Rep. 2017, 7, 8325.
- Guo, Y.; Li, J.; Islam, M.S.; Yan, T.; Zhou, Y.; Liang, L.; Connerton, I.F.; Deng, K.; Li, J. Application of a Novel Phage VB_SalS-LPSTLL for the Biological Control of Salmonella in Foods. Food Res. Int. 2021, 147, 110492.
- Mitropoulou, G.; Nikolaou, A.; Santarmaki, V.; Sgouros, G.; Kourkoutas, Y. Citrus MedicaandCinnamomum ZeylanicumEssential Oils as Potential Biopreservatives against Spoilage in Low Alcohol Wine Products. Foods 2020, 9, 577.
- Campion, A.; Morrissey, R.; Field, D.; Cotter, P.D.; Hill, C.; Ross, R.P. Use of Enhanced Nisin Derivatives in Combination with Food-Grade Oils or Citric Acid to Control Cronobacter Sakazakii and Escherichia coli O157:H7. Food Microbiol. 2017, 65, 254–263.
- Beristain-Bauza, S.; Martinez-Nino, A.; Ramirez-Gonzalez, A.P.; Avila-Sosa, R.; Ruiz-Espinosa, H.; Ruiz-Lopez, I.I.; Ochoa-Velasco, C.E. Inhibition of Salmonella Typhimurium Growth in Coconut (Cocos Nucifera L.) Water by Hurdle Technology. Food Control 2018, 92, 312–318.
- Chung, D.; Cho, T.J.; Rhee, M.S. Citrus Fruit Extracts with Carvacrol and Thymol Eliminated 7-Log Acid-Adapted Escherichia Coli 0157:H7, Salmonella Typhimurium, and Listeria Monocytogenes: A Potential of Effective Natural Antibacterial Agents. Food Res. Int. 2018, 107, 578–588.
- Charfi, S.; Boujida, N.; El Moussaoui, N.; Abrini, J.; Senhaji, N.S. Thymbra Capitata Essential Oil Use to Preserve Physicochemical and Microbiological Qualities of Pomegranate Juice. Food Sci. Technol. Res. 2019, 25, 257–263.
- Boukhatem, M.N.; Boumaiza, A.; Nada, H.G.; Rajabi, M.; Mousa, S.A. Eucalyptus Globulus Essential Oil as a Natural Food Preservative: Antioxidant, Antibacterial and Antifungal Properties in Vitro and in a Real Food Matrix (Orangina Fruit Juice). Appl. Sci. 2020, 10, 5581.
- Dhanda, S.; Kumar, P.; Bansal, P.; Singh, J.; Dhanda, S. Identification, Purification, Characterization and Biopreservation Potential of Antimicrobial Peptide of Pediococcus Acidilactici NCDC 252. Int. J. Pept. Res. Ther. 2023, 29, 15.
- Mitropoulou, G.; Bardouki, H.; Vamvakias, M.; Panas, P.; Paraskevas, P.; Kourkoutas, Y. Assessment of Antimicrobial Efficiency of Pistacia Lentiscus and Fortunella Margarita Essential Oils against Spoilage and Pathogenic Microbes in Ice Cream and Fruit Juices. Microbiol. Res. 2022, 13, 667–680.
- Tenea, G.N.; Barrigas, A. The Efficacy of Bacteriocin-Containing Cell-Free Supernatant from Lactobacillus Plantarum Cys5-4 to Control Pathogenic Bacteria Growth in Artisanal Beverages. Int. Food Res. J. 2018, 25, 2131–2137.
- Friedman, M.; Levin, C.E.; Henika, P.R. Addition of Phytochemical-Rich Plant Extracts Mitigate the Antimicrobial Activity of Essential Oil/Wine Mixtures against Escherichia Coli O157:H7 but Not against Salmonella Enterica. Food Control 2017, 73, 562–565.
- Sanchez-Rubio, M.; Taboada-Rodriguez, A.; Cava-Roda, R.; Lopez-Molina, D.; Marin-Iniesta, F. Combined Use of Thermo-Ultrasound and Cinnamon Leaf Essential Oil to Inactivate Saccharomyces Cerevisiae in Culture Broth and Natural Orange Juice. J. Food Sci. Technol. Mysore 2018, 55, 4623–4633.
- Pedrosa, G.T.D.; de Souza, E.L.; de Melo, A.N.F.; Almeida, E.T.D.; Guedes, J.P.D.; de Carvalho, R.J.; Pagan, R.; Magnani, M. Physiological Alterations Involved in Inactivation of Autochthonous Spoilage Bacteria in Orange Juice Caused by Citrus Essential Oils and Mild Heat. Int. J. Food Microbiol. 2020, 334, 108837.
- Wang, H.; Sun, H. Assessment of Different Antimicrobials to Inhibit the Growth of Zygosaccharomyces Rouxii Cocktail in Concentrated Apple Juice. Food Microbiol. 2020, 91, 103549.
- Bai, H.; Zhou, D.G.; Zhang, X.W.; Cao, Y.F.; Xiao, X.L.; Zhang, Y.; Yu, Y.G. The Responses of Salmonella Enterica Serovar Typhimurium to Vanillin in Apple Juice through Global Transcriptomics. Int. J. Food Microbiol. 2021, 347, 109189.
- Kim, J.; Kim, H.; Beuchat, L.R.; Ryu, J.-H. Synergistic Antimicrobial Activities of Plant Essential Oils against Listeria Monocytogenes in Organic Tomato Juice. Food Control 2021, 125, 108000.
- Wang, H.X.; Peng, Z.H.; Sun, H.M. Antifungal Activities and Mechanisms of Trans-Cinnamaldehyde and Thymol against Food-Spoilage Yeast Zygosaccharomyces Rouxii. J. Food Sci. 2022, 87, 1197–1210.
- Sumonsiri, N. Effect of Nisin on Microbial, Physical and Sensory Qualities of Micro-Filtered Coconut Water (Cocos Nucifera L.) During Refrigerated Storage. Curr. Res. Nutr. Food Sci. 2019, 7, 236–243.
- Mok, J.H.; Pyatkovskyy, T.; Yousef, A.; Sastry, S.K. Synergistic Effects of Shear Stress, Moderate Electric Field, and Nisin for the Inactivation of Escherichia coli K12 and Listeria Innocua in Clear Apple Juice. Food Control 2020, 113, 107209.
- Zhao, Q.Y.; Yuan, Q.Y.; Gao, C.X.; Wang, X.Y.; Zhu, B.H.; Wang, J.Q.; Sun, X.Y.; Ma, T.T. Thermosonication Combined with Natural Antimicrobial Nisin: A Potential Technique Ensuring Microbiological Safety and Improving the Quality Parameters of Orange Juice. Foods 2021, 10, 1851.
- Arulrajah, B.; Qoms, M.S.; Muhialdin, B.J.; Hasan, H.; Zarei, M.; Hussin, A.S.M.; Chau, D.M.; Saari, N. Antibacterial and Antifungal Activity of Kenaf Seed Peptides and Their Effect on Microbiological Safety and Physicochemical Properties of Some Food Models. Food Control 2022, 140, 109119.
- Sun, J.; Gao, Y.; Zhu, X.; Lu, Z.; Lu, Y. Enhanced Antimicrobial Activity against Alicyclobacillus Acidoterrestris in Apple Juice by Genome Shuffling of Lactobacillus Acidophilus NX2-6. J. Food Saf. 2022, 42, e12970.
- Valera, M.J.; Sainz, F.; Mas, A.; Torija, M.J. Effect of Chitosan and SO2 on Viability of Acetobacter Strains in Wine. Int. J. Food Microbiol. 2017, 246, 1–4.
- Paulin, M.; Miot-Sertier, C.; Dutilh, L.; Brasselet, C.; Delattre, C.; Pierre, G.; Dubessay, P.; Michaud, P.; Doco, T.; Ballestra, P.; et al. +Brettanomyces bruxellensis Displays Variable Susceptibility to Chitosan Treatment in Wine. Front. Microbiol. 2020, 11, 571067.
- De Oliveira, E.F.; Nguyen, C.H.; Stepanian, K.; Cossu, A.; Nitin, N. Enhanced Bacterial Inactivation in Apple Juice by Synergistic Interactions between Phenolic Acids and Mild Food Processing Technologies. Innov. Food Sci. Emerg. Technol. 2019, 56, 108613.
- Kim, H.W.; Rhee, M.S. Combined Treatment of β-Resorcylic Acid and Capric Acid Enhances Mild Heat Pasteurization for Inactivating Salmonella Typhimurium in Orange Juice. Int. J. Food Microbiol. 2020, 324, 108613.
- Li, J.; Zhao, N.; Xu, R.Y.; Li, G.M.; Dong, H.Y.; Wang, B.Y.; Li, Z.C.; Fan, M.T.; Wei, X.Y. Deciphering the Antibacterial Activity and Mechanism of P-Coumaric Acid against Alicyclobacillus Acidoterrestris and Its Application in Apple Juice. Int. J. Food Microbiol. 2022, 378, 109822.
- Ruiz-Rico, M.; Pérez-Esteve, É.; Bernardos, A.; Sancenón, F.; Martínez-Máñez, R.; Marcos, M.D.; Barat, J.M. Enhanced Antimicrobial Activity of Essential Oil Components Immobilized on Silica Particles. Food Chem. 2017, 233, 228–236.
- Peña-Gómez, N.; Ruiz-Rico, M.; Fernández-Segovia, I.; Barat, J.M. Study of Apple Juice Preservation by Filtration through Silica Microparticles Functionalised with Essential Oil Components. Food Control 2019, 106, 106749.
- Ribes, S.; Ruiz-Rico, M.; Pérez-Esteve, É.; Fuentes, A.; Talens, P.; Martínez-Máñez, R.; Barat, J.M. Eugenol and Thymol Immobilised on Mesoporous Silica-Based Material as an Innovative Antifungal System: Application in Strawberry Jam. Food Control 2017, 81, 181–188.
- Silva, R.R.; Avelino, K.Y.; Ribeiro, K.L.; Franco, O.L.; Oliveira, M.D.; Andrade, C.A. Chemical Immobilization of Antimicrobial Peptides on Biomaterial Surfaces. Front. Biosci. Sch. 2016, 8, 129–142.
- Rosner, D.; Clark, J. Formulations for Bacteriophage Therapy and the Potential Uses of Immobilization. Pharmaceuticals 2021, 14, 359.
- Ribes, S.; Ruiz-Rico, M.; Perez-Esteve, E.; Fuentes, A.; Barat, J.M. Enhancing the Antimicrobial Activity of Eugenol, Carvacrol and Vanillin Immobilised on Silica Supports against Escherichia Coli or Zygosaccharomyces Rouxii in Fruit Juices by Their Binary Combinations. LWT-Food Sci. Technol. 2019, 113, 108326.
- Liu, Y.; Li, X.; Sheng, J.; Lu, Y.; Sun, H.; Xu, Q.; Zhu, Y.; Song, Y. Preparation and Enhanced Antimicrobial Activity of Thymol Immobilized on Different Silica Nanoparticles with Application in Apple Juice. Coatings 2022, 12, 671.
- Ruiz-Rico, M.; Sancenón, F.; Barat, J.M. Evaluation of the in Vitro and in Situ Antimicrobial Properties of Chitosan-Functionalised Silica Materials. LWT 2023, 173, 114373.
- Song, Z.; Wu, H.; Niu, C.; Wei, J.; Zhang, Y.; Yue, T. Application of Iron Oxide Nanoparticles @ Polydopamine-Nisin Composites to the Inactivation of Alicyclobacillus Acidoterrestris in Apple Juice. Food Chem. 2019, 287, 68–75.
- Ruiz-Rico, M.; Barat, J.M. Natural Antimicrobial-Coated Supports as Filter Aids for the Microbiological Stabilisation of Drinks. LWT 2021, 147, 111634.
- Zhang, J.; Li, S.; Wang, W.; Pei, J.; Zhang, J.; Yue, T.; Youravong, W.; Li, Z. Bacteriocin Assisted Food Functional Membrane for Simultaneous Exclusion and Inactivation of Alicyclobacillus Acidoterrestris in Apple Juice. J. Memb. Sci. 2020, 618, 118741.
- Ruiz-Rico, M.; García-Ríos, E.; Barat, J.M.; Guillamón, J.M. Microbial Stabilisation of White Wine by Filtration through Silica Microparticles Functionalised with Natural Antimicrobials. LWT 2021, 149, 111783.
This entry is offline, you can click here to edit this entry!
