A recent critical review paper notes that growth of Legionella pneumophila and other opportunistic pathogens (OPs) in drinking water premise plumbing poses an increasing public health concern. Premise plumbing is constructed of a variety of materials, creating complex environments that vary chemically, microbiologically, spatially, and temporally in a manner likely to influence survival and growth of OPs. Plastic pipes can leach organic carbon, but demonstrate a lower disinfectant demand and fewer water chemistry interactions. Iron pipes may provide OPs with nutrients directly or indirectly, exhibiting a high disinfectant demand and potential to form scales with high surface areas suitable for biofilm colonization. While copper pipes are known for their antimicrobial properties, evidence of their efficacy for OP control is inconsistent. Under some as yet not fully understood circumstances, copper’s interactions with premise plumbing water chemistry and resident microbes can encourage growth of OPs. Plumbing design, configuration, and operation can be manipulated to control such interactions and health outcomes. Influences of pipe materials on OP physiology should also be considered, including the possibility of influencing virulence and antibiotic resistance. In conclusion, all known pipe materials have a potential to either stimulate or inhibit OP growth, depending on the circumstances. The peer reviewed paper delineates some of these circumstances and informs future research and guidance towards effective deployment of pipe materials for control of OPs.
- Legionnaires’ Disease
- Premise Plumbing Pipe
- opportunistic pathogens
The introduction to the open access peer reviewed paper by Cullom, Martin, Song, Williams, Williams, Pruden and Edwards states the following.
Legionnaires’ Disease is the “leading cause of reportable waterborne illness” in the United States [1,2], with 52,000–70,000 cases per year [1,3,4], 8000–18,000 hospitalizations [5], an overall mortality rate of 15% [4], and high healthcare and legal costs [2,6,7,8]. Bacteria belonging to the genus Legionella are the causative agent of Legionnaires’ disease and Pontiac Fever, which infect the human respiratory system via inhalation or aspiration. Legionella is classified as “opportunistic” because it preferentially infects those with underlying illnesses or weakened immune systems [4,8,9]. To date more than 60 Legionella species have been identified [10], with Legionella pneumophila being the species most commonly attributed to human disease [11]. Legionella can be found even in “the most aggressively treated drinking water” [12]. Studies have confirmed that potable water is a key source of infection [1,4,13,14,15,16,17], for both hospital- and community-acquired cases [18,19,20]. Other opportunistic pathogens (OPs) such as nontuberculous mycobacteria (NTM), Pseudomonas aeruginosa, and Acanthamoebae, can similarly be transmitted via tap water and tend to infect individuals belonging to certain risk groups [8].
To infect humans, Legionella and other OPs must be present in tap water at the point of use. While Legionella can occasionally survive drinking water treatment and be transported through the main water distribution system, the primary environment for Legionella proliferation to numbers needed to infect humans generally occurs in building or “premise” plumbing [21,22]. Premise plumbing includes the service pipe that connects buildings to the water main, in addition to the full array of components comprising cold and hot portions of a building’s potable water system [8]. Premise plumbing is characterized by high surface area to volume ratios, longer stagnation times, low disinfectant residual, areas with excess sediment and scale, chemically and biologically reactive plumbing materials, and water with relatively warm temperatures. Such conditions can create ideal micro- and macro-environmental niches for growth of various OPs [1,8,23].
Premise plumbing is a key conduit for human exposure via showering, handwashing, and other applications that create airborne aerosols [24]. Legionella has been detected in faucets, showerheads, decorative fountains, grocery store mist systems, ice machines, and cooling towers [13,14,16,25]. Larger buildings with more complex plumbing systems are more likely to create physicochemical conditions suited for Legionella proliferation, but it is also often detectable in water mains and residences with simple conventional hot and cold water plumbing systems [17,26,27]. A Centre for Disease Control (CDC) summary of Legionnaires’ Disease potable water outbreak investigations from 2000–2014, concluded that 85% of the cases had “deficiencies” in water system maintenance within buildings as a contributing factor [28] and that water chemistry flowing into buildings is one, but not the only, predictor of Legionella incidence [29,30].
The mechanisms by which premise plumbing influences L. pneumophila and other OPs, as well as the broader premise plumbing microbiome, are varied and complex (Figure 1). The influent water chemistry has been found to influence Legionella, and also strongly shape the plumbing microbiome, especially through the delivery of growth-promoting nutrients, growth-inhibiting disinfectants, and influent microorganisms [31,32,33,34]. The ecological interactions among microorganisms in biofilms of building plumbing systems can also help overcome barriers to growth from low nutrient levels and disinfectants [24,35,36]. Conversely, other interactions, such as competition, exclusion, predation, or inactivation of symbiotic organisms, may inhibit the growth of OPs [37]. The selective pressures in premise plumbing might also alter the physiologies of resident microbes in a manner that influences infectivity [38]. All these phenomena are further complicated by the fact that premise plumbing configurations, hydraulics, temperature, and water use patterns including velocity, flow or stagnation events, all differ significantly from building to building. In particular, there is strong variability due to occupancy, building size, water heater design, water saving devices, storage and other factors [39,40]. Thus, while there are many overarching similarities, every premise plumbing system is at least as variable as the occupants’ unique water use patterns and habits.
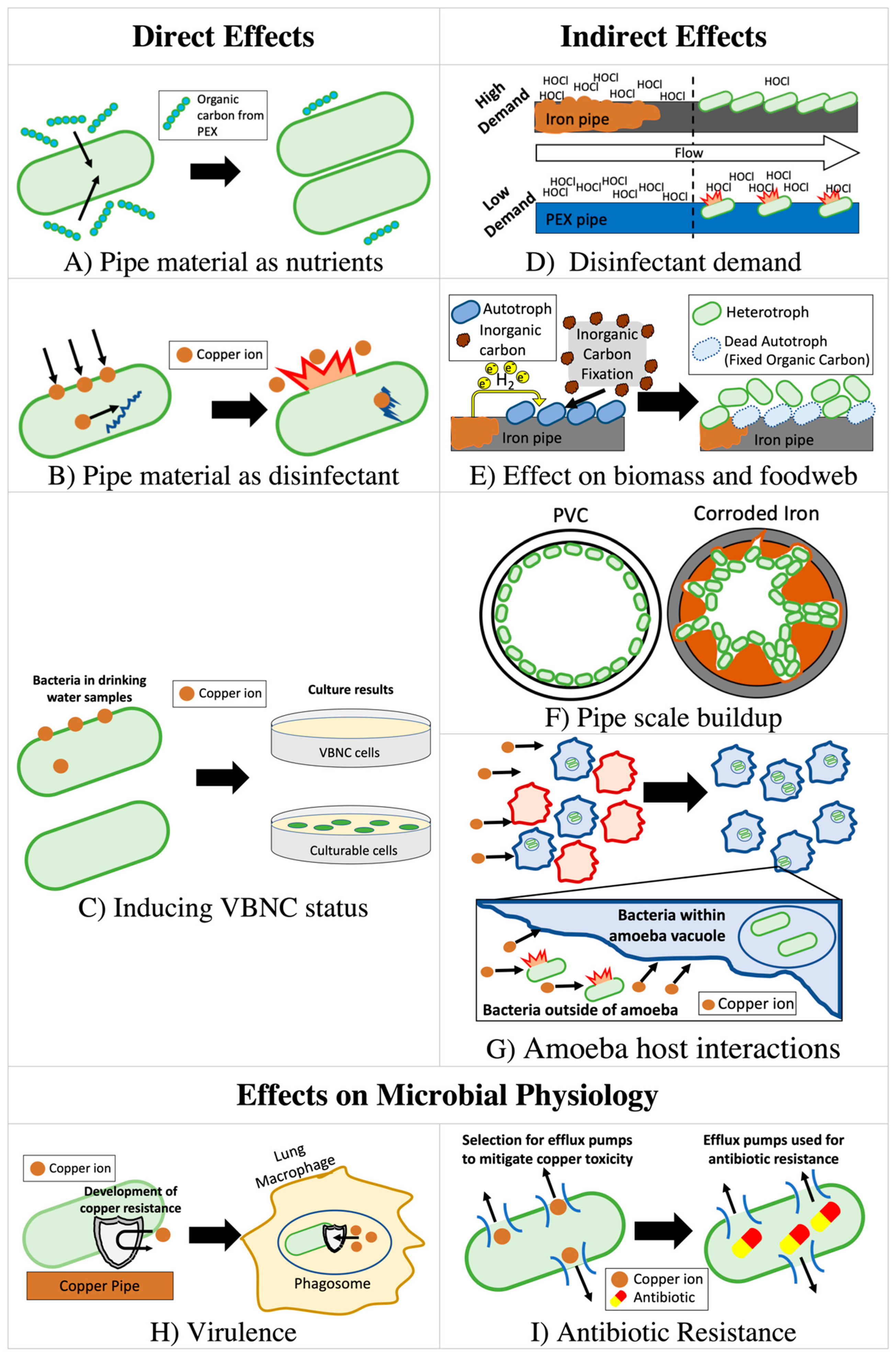
The type of pipe material can also strongly influence the relationship between premise plumbing materials and OPs through both direct effects (interaction with chemical species released from pipe) and indirect effects (secondary consequences of released material from pipes) by altering the level of nutrients, disinfectants, and microbial biomass (Figure 1). Selection of pipe material can therefore strongly affect chemistry, biological stability [41], and microbiome composition [42] of the drinking water.
2. Mediating Role of Microbiome and other Microbiological Considerations
2.1. The Role of Pipe Material in Shaping the Premise Plumbing Microbiome and Resident Amoeba Host Organisms
Interactions between OPs and the microbial communities surrounding them are key to OP proliferation and are likely influenced by pipe materials. OPs can be parasitic to free-living amoebae that first prey upon them in drinking water biofilms, before they reproduce inside and eventually kill the host organism [24]. In fact, there is some doubt that Legionella actually reproduces significantly in drinking water outside of an amoeba host [283]. Amoebae can also protect OPs from disinfectants and provide access to nutrients. For example, Legionella exclusively use amino acids, which are abundant in amoeba vacuoles, as a carbon source [210,211,212,213,214,284,285]. Thus, although poorly studied, any factor altering growth of key host amoebae (including Acanthamoeba, Vermamoeba, and Naegleria) is expected to indirectly affect growth of OPs, including L. pneumophila, P. aeruginosa, and NTM [122,210,211,212,213,214,225,257,286,287]. In one experiment, copper coupons were found to host more Acanthamoeba polyphaga than PVC coupons [288], possibly because copper hosts less diverse eukaryotic communities [64,289] and limits competition for A. polyphaga. As a result, L. pneumophila grew and shed to the bulk waters in higher numbers on these copper coupons than on PVC coupons if co-inoculated with A. polyphaga [122].
Interbacterial interactions may also influence the growth of OPs. Broadly speaking, OPs benefit from the biofilm community through access to nutrients and protection from disinfectants [24,35,36,290]. Some studies have identified correlations between specific taxa and OPs in premise plumbing [291], cooling towers [292] and drinking water distribution systems [293]. However, the significance of these correlations to premise plumbing material selection is not well understood, as most studies examining differences in bacterial communities focus on very broad measures of community structure [48,59,64,216,289,294,295,296]. Certain waterborne bacteria are known to produce toxins that inhibit L. pneumophila growth [216,297] or exude other compounds that have secondary bacteriostatic effects on Legionella [298]. Intra-bacterial inhibition also may be mediated through amoebae by reducing host uptake [299,300] or killing the host population [134,301,302]. More research is needed to elucidate how the broad ecological differences resulting from pipe material influence these interactions. Integration of metagenomic or meta-transcriptomic analyses targeting the production of bacteriocins or other toxins with known effects on OPs could elucidate the ecological effects of taxonomic shifts resulting from pipe material. Interrupting OP-amoeba endosymbiosis through the enrichment of preferential non-OP amoeba prey [299,300] has been suggested as a probiotic means of controlling OPs [303], and pipe material could be explored as a means of enrichment of these taxa.
2.2. Variation in Copper Tolerance Among Species and Strains
Strain-to-strain differences in intrinsic tolerance of copper, acclimation to copper concentrations with time through induction of the appropriate genes, or acquisition of copper resistance via mutation or horizontal gene transfer in premise plumbing might explain some of the discrepancies in variable outcomes of copper on OPs (Table 2). Legionella [155] and other OPs [58] may acclimate to high copper levels through the expression of copper detoxification or efflux systems. Bedard et al. [155] reported four-fold differences in the copper tolerance of environmentally-isolated L. pneumophila strains, noting that more resistant strains showed increased copper ATPase copA expression, speculating that their increased tolerance may also be a result of higher biofilm production. Strikingly, Williams et al. [58] showed that, during exposure to 95 mg/L of copper over 6 h in liquid culture, culturable A. baumannii levels (CFU/mL) could increase by 2-logs or decrease by 2-logs, depending on the strain. The authors identified putative copper detoxification and efflux systems within the genome of the most resistant isolate and identified specific genes that were upregulated in response to copper exposure.
2.3. Confounding Effects of VBNC Bacteria
The discovery of VBNC bacteria has complicated prior understanding for all OP control strategies, including copper. Virtually all prior work relied on culture methods to determine copper’s efficacy for killing OPs [62,63,83,91,92,98,108,109,120,137,153], but some microbes rendered not culturable might remain viable and still infect host amoebae or humans [74,76,305,306,307]. The existence of VBNC pathogens in premise plumbing has been demonstrated by comparing culture-based numbers with those enumerated via fluorescence (e.g., live/dead) and molecular-based (e.g., quantitative polymerase chain reaction) monitoring methods [308].
Bench-scale studies examining copper’s antimicrobial efficacy have found discrepancies between culture-based and molecular-based numbers of L. pneumophila [121,122] that are also suggestive of a copper-induced VBNC state. Similar discrepancies have been noted for P. aeruginosa, Stenotrophomonas maltophilia, and M. avium [104,109,127,132,133]. Evidence of copper-induced VBNC activity is particularly strong in the case of P. aeruginosa, where one study applied multiple non-culture-based measures of viability [127,132]. Furthermore, VBNC P. aeruginosa have been shown to partially recover infectivity after removal of copper from solution [132,133]. To understand how VBNC bacteria contribute to OP infections, additional studies are needed to delineate the premise plumbing conditions more precisely that induce VBNC status and to confirm the range of functionality maintained in this state. A primary challenge in achieving this is that there are currently no reliable methods for confidently enumerating VBNC bacteria.
2.4. Virulence
The premise plumbing environment exhibits several features that could possibly contribute to the virulence of resident OPs. Wargo [38] describes features of drinking water plumbing that could prime OPs to infect cystic fibrosis patients, although the interactions described in this review could also pose risk to otherwise immunocompromised individuals. Such features that are relevant to pipe material include [38]:
- Elevated copper levels, selecting for resistance to copper overload within macrophage phagosomes, a component of the innate immune response [309].
- Elevated iron levels, influencing interactions between iron homeostasis and virulence.
- Exposure to lipids, which are generally not well removed by drinking water treatment, priming OPs for lipid-rich environments within hosts. Accumulation of phospholipid fatty acids has been shown to be greater in the biofilms of polyethylene pipes than copper pipes, though these lipids were putatively associated with bacteria [310].
- Low DO levels, selecting for OPs capable of survival in low DO regions of the biofilm in infected host tissue.
- Exposure to eukaryotic predation, selecting for resistance to the host’s immune response (e.g., lung macrophages) or enhanced virulence.
2.5. Antibiotic Resistance and Tolerance
Copper, among other heavy metals has been shown to exert selection pressure, leading to enhanced survival of antibiotic resistant bacteria. In fact, heavy-metal-associated co-selection and cross-selection has been proposed to be as much of a concern for environmental propagation of antibiotic resistance as antibiotics themselves [322]. Increases in antibiotic resistance genes at the community scale have been identified after long-term copper exposure in soil [323,324,325,326], sediment [327], and drinking water [327]. Bench-scale tests using bacterial isolates from biofilters [328] and wastewater [329] inoculated into growth media have shown that a selective or inductive effect of copper can take places within hours. However, these studies were performed with copper concentrations 5–77 times greater than the 1.3 mg/L US EPA copper action level and similarly in exceedance of the Chinese Standard for Drinking Water Quality of 1 mg/L [209] and WHO Guideline for Drinking-Water Quality of 2 mg/L [82]. Thus, these concentrations may not be representative of potable water systems. One study examining antibiotic resistant and sensitive strains of Staphylococcus aureus showed that the more antibiotic resistant strain survived longer in a copper container [90]. As discussed above, copper may also better support Acanthamoeba than other materials, while in one study L. pneumophila grown within A. polyphaga demonstrated increased tolerance to all antibiotics tested (rifampin, ciprofloxacin, and erythromycin) compared to those grown in culture media [330]. The role of copper plumbing and other pipe materials in these emerging areas of research is worthy of further investigation.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens9110957
