Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Breast cancer is the most frequently diagnosed malignancy worldwide and the leading cause of cancer-related death among women. Brain metastases are a primary contributor to mortality, as they often go undetected until late stages due to their dormant nature. Moreover, the clinical management of brain metastases is complicated by the relevant issue of blood-brain barrier penetration.
- breast cancer
- metastatic breast cancer
- brain metastasis
1. Introduction
Breast cancer (BC) is the most frequently diagnosed malignancy worldwide and the leading cause of cancer-related death in the female population [1]. Significant improvements in diagnosis and treatment strategies have substantially enhanced the management of these patients [2]. However, when the tumors have progressed to an advanced stage, the outcome remains poor, with a 5-year relative survival rate of 31% for de-novo metastatic BC (MBC) [3][4]. The most common anatomical sites of BC metastatic deposits include bone, liver, lung, and brain, with the latter being one of the major causes of mortality [5][6][7]. Of note, brain metastases (BMs) are more frequent in patients with HER2+ or triple-negative BC (TNBC) [3][6][8].
Patients with intracranial disease require multidisciplinary therapeutic approaches, including surgery, radiation, and pharmacological therapies [9][10]. Among these, medical treatments are particularly challenging due to the presence of the blood–brain barrier (BBB), which may limit the successful access to the central nervous system (CNS) of bioactive compounds [11]. Due to the complex crosstalk between the tumor and neural microenvironment, it is paramount to dissect the molecular mechanisms of BC with BMs. This would allow for the optimization of the existing systemic treatments and the critical development of novel and more effective strategies.
2. Biological Mechanisms of Brain Metastasis
Systemic metastases are the result of a multi-stage process that involves the detachment of the neoplastic cells from the primary tumor mass, and their migration through the local mesenchyme into the blood and lymphatic vessels [12]. This process requires reciprocal interactions between tumor cells and host tissues that involve epithelial-to-mesenchymal transition (EMT), changes in adhesion, proteolysis, invasion, and angiogenesis [13][14].
In BC, these events are linked to the alteration of the Wnt signaling pathway and inactivation of cadherin 1 (CDH1) [15][16][17]. Once neoplastic cells reach the peritumoral microenvironment, their survival relies on the so-called intravasation, which is the ability to invade the systemic circulation and reach distant anatomical sites [18]. The brain is a richly vascularized organ and is therefore exposed to a high amount of circulating tumor cells, provided that they can cross the BBB [19]. This barrier is composed of a layer of specialized endothelial cells expressing a specific subset of membrane transporters and pumps that allow tight regulation of the brain microenvironment and are impermeable to most foreign agents [20]. Astrocytes further contribute to the regulation of the BBB by forming a second basement membrane around brain capillaries through their cytoplasmic foot processes [21]. Still, some BC cells can cross the BBB and invade the brain parenchyma.
Colonization of the brain by BC starts with the adhesion of the circulating tumor cells to the capillary endothelium [22][23]. Upregulation of the membrane glycosyl-transferase ST6GALNAC5 has been demonstrated to play an important role in this process [24]. Its expression is normally restricted to the brain, but BC neoplastic cells that acquire the ability to synthesize it have an increased ability to cross the BBB and invade the brain parenchyma [25]. In addition, β4 integrin signaling promotes tumor endothelial adhesion and extravasation by enhancing vascular endothelial growth factor (VEGF) expression, activated by hypoxia, which promotes vascular remodeling and increased permeability [26][27][28]. After the BBB has been breached, reactive astrocytes activated by contact with cancer cells initiate an anti-tumoral response by secreting plasminogen activators [29]. In the early phases of the anti-tumoral response, this promotes the activation of plasmin, which in turn is responsible for the elimination of neoplastic cells [30]. However, some tumor cells can escape by producing anti-plasminogen activator serpins [31]. In the later phases of metastasis growth, reactive astrocytes have a tumor-promoting effect through the creation of a microenvironment that favors metastasis progression [32]. The summary of BC BM biological mechanisms is represented in Figure 1.
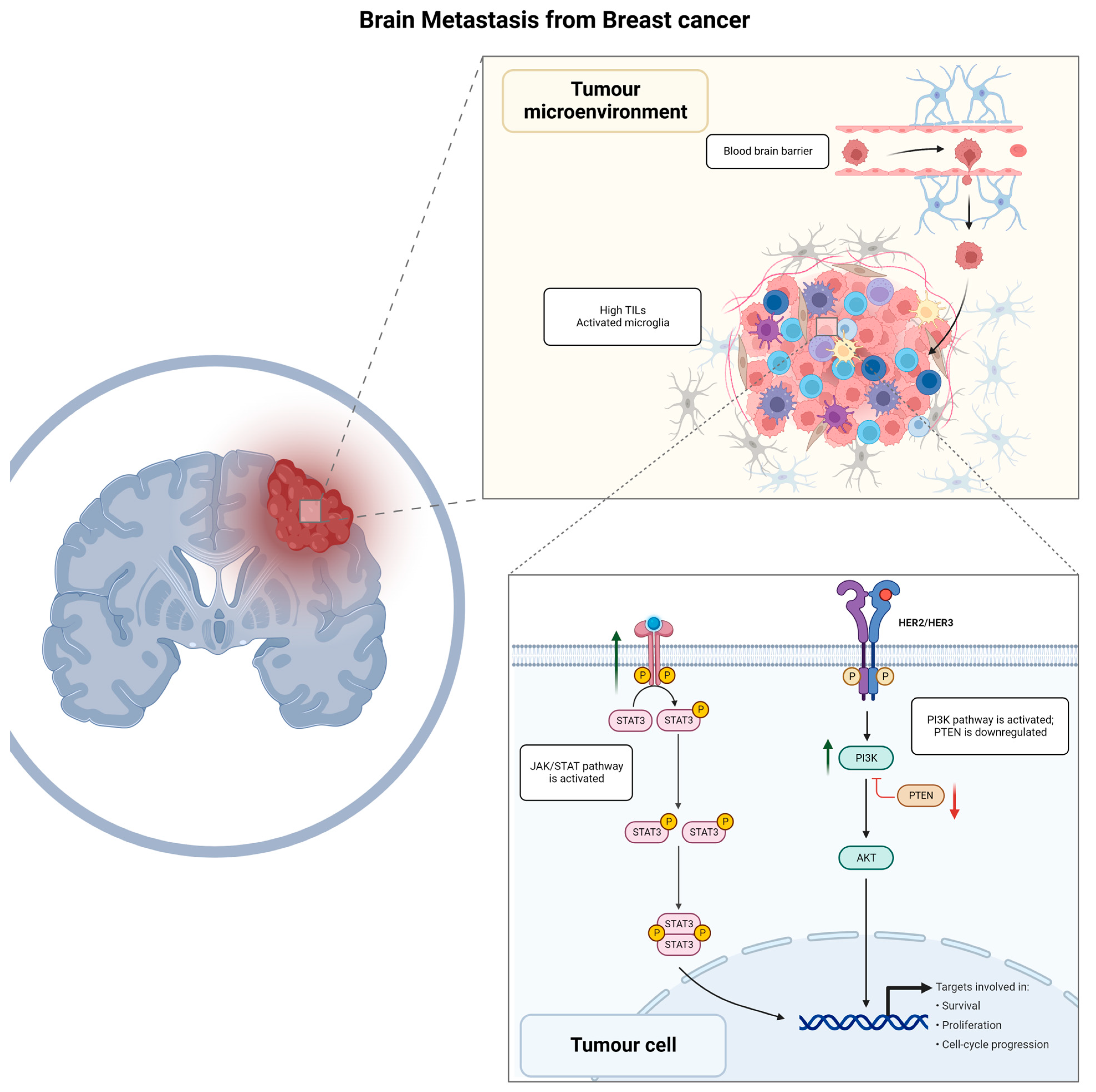
Figure 1. Graphical summary of biological mechanisms involved in brain metastasis development for breast cancer patients. TILs, tumor infiltrating lymphocytes; JAK/STAT, Janus kinase signal transducer and activator of transcription; HER2/HER3, human epidermal growth factor receptor 2/human epidermal growth factor receptor 3; PI3K/AKT/PTEN, phosphoinositide 3—kinase-protein kinase B (Akt)—phosphatase and tensin homolog.
2.1. JAK/STAT Signaling Pathway
Reactive astrocytes following BM are characterized by activation of the JAK/STAT signaling pathway that promotes tissue repair and scar formation following brain injury [33]. Astrocytes expressing pSTAT3 increase the recruitment of CD74+ microglia-macrophages, which in turn induces the activation of the macrophage migration inhibitory factor (MIF) [34][35]. The activation of the CD74-MIF axis has an immunosuppressive effect on peritumoral microglia macrophages, creating a metastasis-favorable microenvironment [36]. In addition, STAT3+ astrocytes have an immune suppressive effect by inducing the expression of programmed cell death–1 (PD-1) and programmed cell death–1 ligand 1 (PD-L1) [37][38][39][40][41]. Furthermore, BC BM has been observed to be highly immunogenic, with high levels of tumor-infiltrating lymphocytes (TILs) frequently reported within the tumor and the surrounding stroma [42][43].
2.2. Immune Checkpoints Mechanisms
A study of 233 patients with solid tumors of various origins and concurrent BM has demonstrated PD-L1 expression in 23.6% of BMs, wherein 18% (19 cases) showed PD-L1 expression in both the primary tumor and the BM (22C3 anti-PD-L1 antibody, Dako Agilent) [44]. Interestingly, authors have also shown that IHC evaluation of CD8 (a co-receptor for the T-cell receptor) resulted in its expression being associated with higher PD-L1 expression, both in the primary tumor and BM, which confirms the ongoing lymphocytic reaction in the BM microenvironment [44]. Significant correlations were identified between the infiltration of CD8-positive lymphocytes in primary tumors and the BM characteristics, with a higher incidence of multiple BMs observed in cases with lower levels of CD8 infiltration in the primary tumors. [44][45]. Griguolo et al. have also confirmed that TNBC BMs showed a significantly higher percentage of intra-tumoral CD8+ cells and a higher density of CD163+ M2-polarized microglia/macrophages within the HER2-negative BC BMs, associating the latter with a worse prognosis, but identifying another potential therapeutic target to be explored [46]. The authors emphasize that, paradoxically, in both TNBC BMs and HR+/HER2− BC BMs, the interaction between CD163+ microglia/macrophages and T lymphocytes was associated with a better outcome [46]. A study by Noh et al. exploring the evolution of the tumor microenvironment in BC BM found that a lower CD8+ T cell count, low CD86+ M1 macrophage count, and high M2/M1 macrophage ratio in the BC BM compared to the primary tumor were related to unfavorable clinical outcomes [47]. Furthermore, Giannoudis et al. have conducted an analysis of 55 samples consisting of 26 paired primary BCs and their BMs, assessing TILs and mRNA expression [48]. Authors have demonstrated a significant reduction of TILs in BC BMs in comparison to primary BCs, with an 11.5% high-TILs count in primary tumors (>40% stromal TILs) versus only 3.8% in BC BMs [48]. A total of 112 immune-related gene levels were found to decrease in BC BMs compared to primary BCs, including PD-L1 and cytotoxic T-lymphocyte antigen 4 (CTLA-4) (false discovery rate < 0.01, log2 fold-change > 1.5), which are involved in cytokine–chemokine signaling, immune cells migration, matrix remodeling, and metastasis [48]. CTLA-4 is one of the fundamental immunosuppressive cytokines, mainly activated on T-cells, which suppresses the immune response, and potentially prevents cancer cells from being attacked by the immune system. CTLA-4 genetic variants have been shown to play a role in BC progression, presenting a prognostic value [49] and making CTLA-4 a potentially attractive target for BC immunotherapeutic approach development, wherein the evaluation of its mutations may become markers for genomics-based precision medicine and effective BC treatment [50]. The depletion of T-cell response could be driven by ARG2 (Arginase 2) upregulation, which was found in BC BMs and confirmed immunohistochemically (ARG2 protein expression was associated with worse breast–brain metastasis-free survival (p = 0.027) and OS (p = 0.019)), so ARG2 could be another potential marker of BC distant metastasis and a therapeutic target in BC BM, as proposed by authors [50]. The model of mouse mammary carcinoma by Sham et al. has provided an analysis of EMT cell lines, wherein tumor cells acquired resistance to radiotherapy, changing their phenotype and causing them to acquire higher migratory and survival rates, which in turn represents a higher metastatic potential [14]. The authors performed a next-generation sequencing (NGS) to explore underlying genes responsible for EMT cell culture and found upregulation of PDL-1, AXL, GAS6, and APCDD1, which are believed to contribute to radioresistance acquisition through the JAK/STAT/PI3K pathway [14]. Based on this hypothesis, the authors determined the levels of PD-1 and CTLA-4 proteins expression, as they are known to be associated with the JAK/STAT/PI3K pathway [51]. Indeed, these proteins were confirmed to be overexpressed in the EMT cell line by Western blot [14]. The immune microenvironment has a profound influence on the outcome of patients with BMS from BC, with a significantly poorer prognosis in tumors with increased PD1/PDL1 expression [46]. However, these patients could benefit from targeted immune therapy [52][53].
2.3. PI3K-Akt Signaling Pathway
The activation of the phosphoinositide 3-kinase (PI3K)-protein kinase B (Akt) signaling pathway has been observed in a large proportion of BCs with BM [54][55]. Activation of this pathway leads to a more aggressive phenotype of neoplastic cells, with increased survivability, proliferation, and angiogenetic potential [56][57]. Patients with BMs harboring activation of the PI3K-Akt signaling pathway have a worse outcome compared to patients with the same-stage disease and lower PI3K-Akt activity [54]. In addition, it contributes to the modulation of the peritumoral immune microenvironment through the activation of immunosuppressive regulators such as PD-1 and CTLA-4 [39]. Interestingly, a functional link has been reported between the PI3K and STAT3, where a synergistic interaction of the two pathways has been observed in murine neoplastic cells [58]. This crosstalk is mediated by the cytoplasmic tyrosine-protein kinase BMX, a member of the TEC kinase family known to be a STAT3 phosphorylator [59][60]. TEC kinases, including BMX, are in turn activated by PI3K, with a positive effect on STAT3 activation [61]. This inter-dependency is further demonstrated by the fact that PI3K inhibition significantly reduces STAT3 phosphorylation and activation [62].
Another important component activating the PI3K-AKT pathway is HER2-HER3 dimerization [63], which plays a central role in the biology of BC with BM [54][57][64][65]. Patients with HER2+ disease are at increased risk of developing BMs [3][8][66], with HER2 overexpression often preserved at the metastatic site, and associated with the overexpression of HER3 [63], a coreceptor that forms heterodimers with HER2, thus playing a role in the neoplastic transformation of HER2-enriched BCs [67]. This provides a further therapeutic perspective on patients with BMs from BC, as HER3 inhibitors are available and have been proven to be effective in increasing the sensitivity to PI3K inhibitors [68].
2.4. PTEN
Another frequent occurrence in BC BM is the loss of phosphatase and tensin homolog (PTEN) [69]. This event has been observed to be more frequent in BM compared to dissemination to other organs, suggesting a role of the local microenvironment in inducing this alteration [70]. This mechanism has indeed been demonstrated to depend on epigenetic regulation through micro-RNAs (miRNAs) secretion by astrocytes [71]. In addition, PTEN loss also induces the secretion of cytokine–chemokine ligand 2 (CCL2), which is responsible for the creation of a pro-inflammatory microenvironment [72], and recruitment of ionized calcium-binding adapter molecule 1 (Iba1)+ myeloid cells that reduce apoptosis and promote growth in neoplastic cells [71].
3. Targeting Therapies for Breast Cancer Patients with Brain Metastasis
According to the latest ASCO-SNO-ASTRO guidelines, surgery with subsequent radiotherapy on the operated field may be offered for patients with one brain mass without primary cancer diagnosis or for large tumors with mass effect. Local treatment with radiotherapy is reasonable for symptomatic BMs, regardless of the systemic therapy administration [73]. There is not one recommended sequence for patients receiving both local treatments and systemic therapy [74]. Medical treatments for BC are mainly based on hormone receptor and HER2 status, while some novel target therapies are available depending on the tumor molecular profile [7][73][75]. Pharmacological approaches for patients harboring encephalic disease do not differ from patients who do not, except for HER2-positive BC, for which guidelines providing a specific treatment algorithm have been provided [76].
3.1. HR+/HER2−—Breast Cancer
Local treatments (surgery and radiation therapy) remain the gold standard for BMs from HR-positive/HER2-negative (HR+/HER2−) BC [73][74]. Existing practice guidelines for MBC treatment recommend sequential endocrine/targeted therapy until the exhaustion of available agents, before systemic cytotoxic chemotherapy administration [74][77][78]. Although BMs in HR+/HER2− BC show a lower incidence compared to HER2+ and TNBC subtypes, endocrine therapy has been found to be beneficial for both CNS and systemic management [74][79]. Indeed, tamoxifen and its metabolites have been reported to achieve effective concentrations in the CNS [80]. First-line treatment for this population is based on endocrine therapy plus cyclin-dependent kinase 4/6 (CDK4/6) inhibitors, namely palbociclib, ribociclib, or abemaciclib [81]. These three agents are capable of crossing the BBB, but there is a lack of clinical data to inform a CNS-specific response rate. Indeed, no data on CNS outcomes are available for ribociclib and palbociclib [74]. Regarding abemaciclib, a single-arm phase II clinical trial did not meet its primary endpoint of intracranial overall response rate in HR-positive BC with BMs, although the drug achieved an excellent concentration in cerebrospinal fluid. It is worth noting that palbociclib has been shown to be effective in BMs of different tumor types, including BC, and to harbor a CDK4/6 alteration [82]. Companion endocrine therapy for CDK4/6 inhibitors could be represented by fulvestrant or letrozole. Elacestrant, an oral selective estrogen receptor degrader approved by the FDA, could be an option for patients harboring an ESR1 mutation after CDK4/6 exposure [83]. However, no data on CNS efficacy are available for this drug at the time of writing.
Furthermore, preclinical data have shown that the serine/threonine protein kinase AKT (protein kinase B) inhibitor capivasertib is active in combination with fulvestrant, providing the basis for future studies [84]. Given the promising results of the phase II FAKTION trial (NCT01992952) [85][86], capivasertib was further investigated in the phase III CAPItello-291 trial (NCT04305496), with results recently reported at the San Antonio Breast Cancer Symposium 2022 [87]. Although patients with BMs were included, results in this subgroup are pending.
Another crucial pathway in BC is the PI3K/Akt/mammalian target of rapamycin (mTOR) signaling, wherein activating PIK3CA mutations, occurring in about 40% of hormone receptor HR+/HER2− MBCs, are driver events for tumorigenesis and tumor progression [57]. This pathway has been successfully targeted by the α-selective PIK3CA inhibitor alpelisib [55], with a manageable safety profile (mainly diarrhea and hyperglycemia). The incidence of BMs is particularly high among patients with HR+/HER2− BC patients carrying a PIK3CA mutation [88], and one report has shown a gain of PIK3CA mutation in BM samples despite its absence in a primary BC [89]. Real-world evidence suggests that alpelisib may have CNS activity [90]. However, the low number of cases reported justifies the need for additional investigations to prospectively evaluate alpelisib efficacy in patients with BC BM [91].
3.2. Triple-Negative Breast Cancer
Among the three main BC subtypes, TNBC is characterized by a limited therapeutic armamentarium [92]. However, this scenario is rapidly expanding thanks to innovative options based on the characterization of individual tumor molecular profiles [93][94][95][96]. At the moment, the treatment strategy for most TNBCs relies on chemotherapy, immunotherapy (if PD-L1 is positive), and target therapy with poly (adenosine diphosphate–ribose) polymerase inhibitors (PARPis) based on germline BRCA1/2 (gBRCA1/2) status. [76][97][98][99]. In patients with MBC harboring a gBRCA1/2 pathogenetic variant, the two PARPis, talazoparib and olaparib, showed a benefit in survival outcomes [100]. Regarding BMs, a higher frequency of BMs is reported in this population. In the EMBRACA trial (NCT01945775), talazoparib resulted in significantly longer progression-free survival than standard-of-care chemotherapy; this was also found in the subgroup of patients with CNS metastases [101]. However, the final overall survival analysis was not statistically significant [102]. It is worth noting that the enrolled population comprised both TNBC and HR+ BC; no specific analysis for BMs in each subgroup is available, given the limited number of patients with BMs enrolled in the study (about 15%). The same concern could be raised for the OlympiAD trial, although in this study no specific subgroup analysis for BMs is available [103]. In these studies, PARPis have shown mainly hematological and gastrointestinal toxicity.
On the other hand, immunotherapy has demonstrated potential intracranial efficacy among patients with MBC [104][105]. Indeed, recent studies demonstrated that TNBCs exhibit higher PD-L1 expression than other BC subtypes, which is associated with remarkable genomic instability and higher immune infiltration [106]. The role of the immune microenvironment and PD-L1 expression in the brain and metastatic brain tumors is poorly understood [46][48]. Chehade and colleagues have studied 59 immunotherapy-naïve BC patients with BMs in a single-center retrospective cohort study, wherein 15.3% had PD-L1+ BM, with the highest proportion (25%) among those with TNBC (SP142 antibody, Ventana) [52]. The concordance in PD-L1 expression between primary BC and BM in TNBC specifically could not be studied due to small sample sizes, but authors emphasize that PD-L1 expression has previously been reported to be higher in the primary tumor compared to metastatic sites (63.7% vs. 42.2%, p < 0.0001), specifically in TNBC [52][107]. Hence, it was proposed that PD-L1 staining should be performed both on the primary and metastatic tumor to maximize the opportunity for ICI therapy administration [107].
Focusing on clinical data, the combination of chemotherapy and pembrolizumab improved overall survival with manageable safety in patients with PD-L1+ (CPS ≥ 10) advanced TNBC in the phase III KEYNOTE-355b study [108]. Only a few patients with BMs were enrolled; thus, it is not possible to derive any conclusion [109][110]. The IMPASSION-130 trial (NCT03483012) has demonstrated a benefit of atezolizumab in combination with nab-paclitaxel for TNBC treatment; however, no progression-free survival (PFS) benefit for BM patients has been observed, although this population had a relatively small representation (6.3%) [111].
This entry is adapted from the peer-reviewed paper 10.3390/genes14061160
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Agostinetto, E.; Gligorov, J.; Piccart, M. Systemic therapy for early-stage breast cancer: Learning from the past to build the future. Nat. Rev. Clin. Oncol. 2022, 19, 763–774.
- Wang, R.; Zhu, Y.; Liu, X.; Liao, X.; He, J.; Niu, L. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer 2019, 19, 1091.
- SEER*Explorer: An Interactive Website for SEER Cancer Statistics. Cancer Stat Facts: Female Breast Cancer. Available online: https://seer.cancer.gov/statistics-network/explorer/ (accessed on 29 April 2023).
- Venetis, K.; Piciotti, R.; Sajjadi, E.; Invernizzi, M.; Morganti, S.; Criscitiello, C.; Fusco, N. Breast Cancer with Bone Metastasis: Molecular Insights and Clinical Management. Cells 2021, 10, 1377.
- Fan, J.-H.; Zhang, S.; Yang, H.; Yi, Z.-B.; Ouyang, Q.-C.; Yan, M.; Wang, X.-J.; Hu, X.-C.; Jiang, Z.-F.; Huang, T.; et al. Molecular subtypes predict the preferential site of distant metastasis in advanced breast cancer: A nationwide retrospective study. Front. Oncol. 2023, 13, 978985.
- Suh, J.H.; Kotecha, R.; Chao, S.T.; Ahluwalia, M.S.; Sahgal, A.; Chang, E.L. Current approaches to the management of brain metastases. Nat. Rev. Clin. Oncol. 2020, 17, 279–299.
- Kuksis, M.; Gao, Y.; Tran, W.; Hoey, C.; Kiss, A.; Komorowski, A.S.; Dhaliwal, A.J.; Sahgal, A.; Das, S.; Chan, K.K.; et al. The incidence of brain metastases among patients with metastatic breast cancer: A systematic review and meta-analysis. Neuro Oncol. 2021, 23, 894–904.
- Wang, Y.; Ye, F.; Liang, Y.; Yang, Q. Breast cancer brain metastasis: Insight into molecular mechanisms and therapeutic strategies. Br. J. Cancer 2021, 125, 1056–1067.
- Ren, D.; Cheng, H.; Wang, X.; Vishnoi, M.; Teh, B.S.; Rostomily, R.; Chang, J.; Wong, S.T.; Zhao, H. Emerging treatment strategies for breast cancer brain metastasis: From translational therapeutics to real-world experience. Ther. Adv. Med. Oncol. 2020, 12, 1758835920936151.
- Mohammad, A.S.; Adkins, C.E.; Shah, N.; Aljammal, R.; Griffith, J.I.G.; Tallman, R.M.; Jarrell, K.L.; Lockman, P.R. Permeability changes and effect of chemotherapy in brain adjacent to tumor in an experimental model of metastatic brain tumor from breast cancer. BMC Cancer 2018, 18, 1225.
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572.
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118.
- Sham, N.F.R.; Hasani, N.A.H.; Hasan, N.; Karim, M.K.A.; Fuad, S.; Hasbullah, H.H.; Ibahim, M.J. Acquired radioresistance in EMT6 mouse mammary carcinoma cell line is mediated by CTLA-4 and PD-1 through JAK/STAT/PI3K pathway. Sci. Rep. 2023, 13, 3108.
- Corso, G.; Figueiredo, J.; La Vecchia, C.; Veronesi, P.; Pravettoni, G.; Macis, D.; Karam, R.; Lo Gullo, R.; Provenzano, E.; Toesca, A.; et al. Hereditary lobular breast cancer with an emphasis on E-cadherin genetic defect. J. Med. Genet. 2018, 55, 431–441.
- Porta, F.M.; Blanco, M.C.; Ivanova, M.; Fusco, N.; Guerini-Rocco, E. Pathology and Somatic Alterations in Hereditary Lobular Breast Cancers. In Hereditary Gastric and Breast Cancer Syndrome: CDH1: One Genotype with Multiple Phenotypes; Corso, G., Veronesi, P., Roviello, F., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 167–180.
- Corso, G.; Montagna, G.; Figueiredo, J.; La Vecchia, C.; Fumagalli Romario, U.; Fernandes, M.S.; Seixas, S.; Roviello, F.; Trovato, C.; Guerini-Rocco, E.; et al. Hereditary Gastric and Breast Cancer Syndromes Related to CDH1 Germline Mutation: A Multidisciplinary Clinical Review. Cancers 2020, 12, 1598.
- Wyckoff, J.B.; Jones, J.G.; Condeelis, J.S.; Segall, J.E. A critical step in metastasis: In vivo analysis of intravasation at the primary tumor. Cancer Res. 2000, 60, 2504–2511.
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25.
- Zhang, S.; Deng, G.; Liu, F.; Peng, B.; Bao, Y.; Du, F.; Chen, A.T.; Liu, J.; Chen, Z.; Ma, J.; et al. Autocatalytic Delivery of Brain Tumor-Targeting, Size-Shrinkable Nanoparticles for Treatment of Breast Cancer Brain Metastases. Adv. Funct. Mater. 2020, 30, 1910651.
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Prim. 2019, 5, 5.
- Invernizzi, M.; Corti, C.; Lopez, G.; Michelotti, A.; Despini, L.; Gambini, D.; Lorenzini, D.; Guerini-Rocco, E.; Maggi, S.; Noale, M.; et al. Lymphovascular invasion and extranodal tumour extension are risk indicators of breast cancer related lymphoedema: An observational retrospective study with long-term follow-up. BMC Cancer 2018, 18, 935.
- Jensen, M.R.; Simonsen, L.; Karlsmark, T.; Bülow, J. Microvascular filtration is increased in the forearms of patients with breast cancer-related lymphedema. J. Appl. Physiol. 2013, 114, 19–27.
- Okajima, T.; Fukumoto, S.; Ito, H.; Kiso, M.; Hirabayashi, Y.; Urano, T.; Furukawa, K. Molecular cloning of brain-specific GD1alpha synthase (ST6GalNAc V) containing CAG/Glutamine repeats. J. Biol. Chem. 1999, 274, 30557–30562.
- Bos, P.D.; Zhang, X.H.; Nadal, C.; Shu, W.; Gomis, R.R.; Nguyen, D.X.; Minn, A.J.; van de Vijver, M.J.; Gerald, W.L.; Foekens, J.A.; et al. Genes that mediate breast cancer metastasis to the brain. Nature 2009, 459, 1005–1009.
- Fan, J.; Cai, B.; Zeng, M.; Hao, Y.; Giancotti, F.G.; Fu, B.M. Integrin β4 signaling promotes mammary tumor cell adhesion to brain microvascular endothelium by inducing ErbB2-mediated secretion of VEGF. Ann. Biomed. Eng. 2011, 39, 2223–2241.
- Yang, X.; Zhang, Y.; Hosaka, K.; Andersson, P.; Wang, J.; Tholander, F.; Cao, Z.; Morikawa, H.; Tegnér, J.; Yang, Y.; et al. VEGF-B promotes cancer metastasis through a VEGF-A-independent mechanism and serves as a marker of poor prognosis for cancer patients. Proc. Natl. Acad. Sci. USA 2015, 112, E2900–E2909.
- Invernizzi, M.; Lopez, G.; Michelotti, A.; Venetis, K.; Sajjadi, E.; De Mattos-Arruda, L.; Ghidini, M.; Runza, L.; de Sire, A.; Boldorini, R.; et al. Integrating Biological Advances into the Clinical Management of Breast Cancer Related Lymphedema. Front. Oncol. 2020, 10, 422.
- Le, D.M.; Besson, A.; Fogg, D.K.; Choi, K.S.; Waisman, D.M.; Goodyer, C.G.; Rewcastle, B.; Yong, V.W. Exploitation of astrocytes by glioma cells to facilitate invasiveness: A mechanism involving matrix metalloproteinase-2 and the urokinase-type plasminogen activator-plasmin cascade. J. Neurosci. 2003, 23, 4034–4043.
- Wasilewski, D.; Priego, N.; Fustero-Torre, C.; Valiente, M. Reactive Astrocytes in Brain Metastasis. Front. Oncol. 2017, 7, 298.
- Valiente, M.; Obenauf, A.C.; Jin, X.; Chen, Q.; Zhang, X.H.; Lee, D.J.; Chaft, J.E.; Kris, M.G.; Huse, J.T.; Brogi, E.; et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 2014, 156, 1002–1016.
- Wu, K.; Fukuda, K.; Xing, F.; Zhang, Y.; Sharma, S.; Liu, Y.; Chan, M.D.; Zhou, X.; Qasem, S.A.; Pochampally, R.; et al. Roles of the cyclooxygenase 2 matrix metalloproteinase 1 pathway in brain metastasis of breast cancer. J. Biol. Chem. 2015, 290, 9842–9854.
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200.
- Leng, L.; Metz, C.N.; Fang, Y.; Xu, J.; Donnelly, S.; Baugh, J.; Delohery, T.; Chen, Y.; Mitchell, R.A.; Bucala, R. MIF signal transduction initiated by binding to CD74. J. Exp. Med. 2003, 197, 1467–1476.
- Sajjadi, E.; Gaudioso, G.; Terrasi, A.; Boggio, F.; Venetis, K.; Ivanova, M.; Bertolasi, L.; Lopez, G.; Runza, L.; Premoli, A.; et al. Osteoclast-like stromal giant cells in breast cancer likely belong to the spectrum of immunosuppressive tumor-associated macrophages. Front. Mol. Biosci. 2022, 9, 894247.
- Ghoochani, A.; Schwarz, M.A.; Yakubov, E.; Engelhorn, T.; Doerfler, A.; Buchfelder, M.; Bucala, R.; Savaskan, N.E.; Eyüpoglu, I.Y. MIF-CD74 signaling impedes microglial M1 polarization and facilitates brain tumorigenesis. Oncogene 2016, 35, 6246–6261.
- Priego, N.; Zhu, L.; Monteiro, C.; Mulders, M.; Wasilewski, D.; Bindeman, W.; Doglio, L.; Martínez, L.; Martínez-Saez, E.; Ramón, Y.; et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat. Med. 2018, 24, 1024–1035.
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201.
- Hosonaga, M.; Saya, H.; Arima, Y. Molecular and cellular mechanisms underlying brain metastasis of breast cancer. Cancer Metastasis Rev. 2020, 39, 711–720.
- Antonarelli, G.; Pieri, V.; Porta, F.M.; Fusco, N.; Finocchiaro, G.; Curigliano, G.; Criscitiello, C. Targeting Post-Translational Modifications to Improve Combinatorial Therapies in Breast Cancer: The Role of Fucosylation. Cells 2023, 12, 840.
- Criscitiello, C.; Guerini-Rocco, E.; Viale, G.; Fumagalli, C.; Sajjadi, E.; Venetis, K.; Piciotti, R.; Invernizzi, M.; Malapelle, U.; Fusco, N. Immunotherapy in Breast Cancer Patients: A Focus on the Use of the Currently Available Biomarkers in Oncology. Anticancer Agents Med. Chem. 2022, 22, 787–800.
- Duchnowska, R.; Pęksa, R.; Radecka, B.; Mandat, T.; Trojanowski, T.; Jarosz, B.; Czartoryska-Arłukowicz, B.; Olszewski, W.P.; Och, W.; Kalinka-Warzocha, E.; et al. Immune response in breast cancer brain metastases and their microenvironment: The role of the PD-1/PD-L axis. Breast Cancer Res. 2016, 18, 43.
- Venetis, K.; Sajjadi, E.; Peccatori, F.A.; Guerini-Rocco, E.; Fusco, N. Immune plasticity in pregnancy-associated breast cancer tumorigenesis. Eur. J. Cancer Prev. 2023.
- Camy, F.; Karpathiou, G.; Dumollard, J.M.; Magne, N.; Perrot, J.L.; Vassal, F.; Picot, T.; Mobarki, M.; Forest, F.; Casteillo, F.; et al. Brain metastasis PD-L1 and CD8 expression is dependent on primary tumor type and its PD-L1 and CD8 status. J. Immunother. Cancer 2020, 8, e000597.
- Zou, W.; Luo, X.; Gao, M.; Yu, C.; Wan, X.; Yu, S.; Wu, Y.; Wang, A.; Fenical, W.; Wei, Z.; et al. Optimization of Cancer Immunotherapy on the Basis of PD-L1 Distribution and Function. Br. J. Pharm. 2023, 1–16.
- Griguolo, G.; Tosi, A.; Dieci, M.V.; Fineberg, S.; Rossi, V.; Ventura, A.; Bottosso, M.; Bauchet, L.; Miglietta, F.; Jacob, J.; et al. A comprehensive profiling of the immune microenvironment of breast cancer brain metastases. Neuro Oncol. 2022, 24, 2146–2158.
- Noh, M.G.; Kim, S.S.; Kim, Y.J.; Jung, T.Y.; Jung, S.; Rhee, J.H.; Lee, J.H.; Lee, J.S.; Cho, J.H.; Moon, K.S.; et al. Evolution of the Tumor Microenvironment toward Immune-Suppressive Seclusion during Brain Metastasis of Breast Cancer: Implications for Targeted Therapy. Cancers 2021, 13, 4895.
- Giannoudis, A.; Varešlija, D.; Sharma, V.; Zakaria, R.; Platt-Higgins, A.; Rudland, P.S.; Jenkinson, M.D.; Young, L.S.; Palmieri, C. Characterisation of the immune microenvironment of primary breast cancer and brain metastasis reveals depleted T-cell response associated to ARG2 expression. ESMO Open 2022, 7, 100636.
- Wu, J.; Li, L.; Chen, J.; Liu, Y.; Xu, J.; Peng, Z. Clinical value of CTLA4 combined with clinicopathological factors in evaluating the prognosis of breast cancer. Gland. Surg. 2020, 9, 1328–1337.
- Chang, H.Y.; Liu, C.Y.; Lo, Y.L.; Chiou, S.H.; Lu, K.H.; Lee, M.C.; Wang, Y.H. Cytotoxic T-lymphocyte antigen 4 polymorphisms and breast cancer susceptibility: Evidence from a meta-analysis. J. Chin. Med. Assoc. 2023, 86, 207–219.
- Le Mercier, I.; Lines, J.L.; Noelle, R.J. Beyond CTLA-4 and PD-1, the Generation Z of Negative Checkpoint Regulators. Front. Immunol. 2015, 6, 418.
- Chehade, R.; Qazi, M.A.; Ennis, M.; Sahgal, A.; Das, S.; Nofech-Mozes, S.; Jerzak, K.J. PD-L1 expression in breast cancer brain metastases. Neuro Oncol. Adv. 2022, 4, vdac154.
- Goldberg, S.B.; Gettinger, S.N.; Mahajan, A.; Chiang, A.C.; Herbst, R.S.; Sznol, M.; Tsiouris, A.J.; Cohen, J.; Vortmeyer, A.; Jilaveanu, L.; et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: Early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 976–983.
- Blazquez, R.; Wlochowitz, D.; Wolff, A.; Seitz, S.; Wachter, A.; Perera-Bel, J.; Bleckmann, A.; Beißbarth, T.; Salinas, G.; Riemenschneider, M.J.; et al. PI3K: A master regulator of brain metastasis-promoting macrophages/microglia. Glia 2018, 66, 2438–2455.
- Fusco, N.; Malapelle, U.; Fassan, M.; Marchiò, C.; Buglioni, S.; Zupo, S.; Criscitiello, C.; Vigneri, P.; Dei Tos, A.P.; Maiorano, E.; et al. PIK3CA Mutations as a Molecular Target for Hormone Receptor-positive, HER2-Negative Metastatic Breast Cancer. Front. Oncol. 2021, 11, 562.
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949.
- Venetis, K.; Pepe, F.; Munzone, E.; Sajjadi, E.; Russo, G.; Pisapia, P.; Ivanova, M.; Bonizzi, G.; Vacirca, D.; Rappa, A.; et al. Analytical Performance of Next-Generation Sequencing and RT-PCR on Formalin-Fixed Paraffin-Embedded Tumor Tissues for PIK3CA Testing in HR+/HER2− Breast Cancer. Cells 2022, 11, 3545.
- Vogt, P.K.; Hart, J.R. PI3K and STAT3: A new alliance. Cancer Discov. 2011, 1, 481–486.
- Guryanova, O.A.; Wu, Q.; Cheng, L.; Lathia, J.D.; Huang, Z.; Yang, J.; MacSwords, J.; Eyler, C.E.; McLendon, R.E.; Heddleston, J.M.; et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell 2011, 19, 498–511.
- Fusco, N.; Guerini-Rocco, E.; Augello, C.; Terrasi, A.; Ercoli, G.; Fumagalli, C.; Vacirca, D.; Braidotti, P.; Parafioriti, A.; Jaconi, M.; et al. Recurrent NAB2-STAT6 gene fusions and oestrogen receptor-alpha expression in pulmonary adenofibromas. Histopathology 2017, 70, 906–917.
- Jiang, X.; Borgesi, R.A.; McKnight, N.C.; Kaur, R.; Carpenter, C.L.; Balk, S.P. Activation of nonreceptor tyrosine kinase Bmx/Etk mediated by phosphoinositide 3-kinase, epidermal growth factor receptor, and ErbB3 in prostate cancer cells. J. Biol. Chem. 2007, 282, 32689–32698.
- Hart, J.R.; Liao, L.; Yates, J.R.; Vogt, P.K. Essential role of Stat3 in PI3K-induced oncogenic transformation. Proc. Natl. Acad. Sci. USA 2011, 108, 13247–13252.
- Lee-Hoeflich, S.T.; Crocker, L.; Yao, E.; Pham, T.; Munroe, X.; Hoeflich, K.P.; Sliwkowski, M.X.; Stern, H.M. A central role for HER3 in HER2-amplified breast cancer: Implications for targeted therapy. Cancer Res. 2008, 68, 5878–5887.
- Adamo, B.; Deal, A.M.; Burrows, E.; Geradts, J.; Hamilton, E.; Blackwell, K.L.; Livasy, C.; Fritchie, K.; Prat, A.; Harrell, J.C.; et al. Phosphatidylinositol 3-kinase pathway activation in breast cancer brain metastases. Breast Cancer Res. 2011, 13, R125.
- Venetis, K.; Crimini, E.; Sajjadi, E.; Corti, C.; Guerini-Rocco, E.; Viale, G.; Curigliano, G.; Criscitiello, C.; Fusco, N. HER2 low, ultra-low, and novel complementary biomarkers: Expanding the spectrum of HER2 positivity in breast cancer. Front. Mol. Biosci. 2022, 9, 834651.
- Sajjadi, E.; Venetis, K.; Piciotti, R.; Gambini, D.; Blundo, C.; Runza, L.; Ferrero, S.; Guerini-Rocco, E.; Fusco, N. Combined analysis of PTEN, HER2, and hormone receptors status: Remodeling breast cancer risk profiling. BMC Cancer 2021, 21, 1152.
- Pinkas-Kramarski, R.; Soussan, L.; Waterman, H.; Levkowitz, G.; Alroy, I.; Klapper, L.; Lavi, S.; Seger, R.; Ratzkin, B.J.; Sela, M.; et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996, 15, 2452–2467.
- Kodack, D.P.; Askoxylakis, V.; Ferraro, G.B.; Sheng, Q.; Badeaux, M.; Goel, S.; Qi, X.; Shankaraiah, R.; Cao, Z.A.; Ramjiawan, R.R.; et al. The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation. Sci. Transl. Med. 2017, 9, eaal4682.
- Fusco, N.; Sajjadi, E.; Venetis, K.; Gaudioso, G.; Lopez, G.; Corti, C.; Rocco, E.G.; Criscitiello, C.; Malapelle, U.; Invernizzi, M. PTEN Alterations and Their Role in Cancer Management: Are We Making Headway on Precision Medicine? Genes 2020, 11, 719.
- Lopez, G.; Noale, M.; Corti, C.; Gaudioso, G.; Sajjadi, E.; Venetis, K.; Gambini, D.; Runza, L.; Costanza, J.; Pesenti, C.; et al. PTEN Expression as a Complementary Biomarker for Mismatch Repair Testing in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1461.
- Zhang, L.; Zhang, S.; Yao, J.; Lowery, F.J.; Zhang, Q.; Huang, W.C.; Li, P.; Li, M.; Wang, X.; Zhang, C.; et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 2015, 527, 100–104.
- Conti, I.; Rollins, B.J. CCL2 (monocyte chemoattractant protein-1) and cancer. Semin. Cancer Biol. 2004, 14, 149–154.
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 2022, 40, 492–516.
- Jusino, S.; Fadul, C.E.; Dillon, P. Systematic review of the management of brain metastases from hormone receptor positive breast cancer. J. Neuro Oncol. 2023, 162, 45–57.
- Huo, X.; Shen, G.; Wang, T.; Li, J.; Xie, Q.; Liu, Z.; Wang, M.; Zhao, F.; Ren, D.; Zhao, J. Treatment options for patients with human epidermal growth factor 2-positive breast cancer brain metastases: A systematic review and meta-analysis. Front. Oncol. 2023, 13, 1003565.
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer☆. Ann. Oncol. 2021, 32, 1475–1495.
- Lin, N.U.; Gaspar, L.E.; Soffietti, R. Breast Cancer in the Central Nervous System: Multidisciplinary Considerations and Management. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 45–56.
- Lopez, G.; Costanza, J.; Colleoni, M.; Fontana, L.; Ferrero, S.; Miozzo, M.; Fusco, N. Molecular Insights into the Classification of Luminal Breast Cancers: The Genomic Heterogeneity of Progesterone-Negative Tumors. Int. J. Mol. Sci. 2019, 20, 510.
- Grizzi, G.; Ghidini, M.; Botticelli, A.; Tomasello, G.; Ghidini, A.; Grossi, F.; Fusco, N.; Cabiddu, M.; Savio, T.; Petrelli, F. Strategies for Increasing the Effectiveness of Aromatase Inhibitors in Locally Advanced Breast Cancer: An Evidence-Based Review on Current Options. Cancer Manag. Res. 2020, 12, 675–686.
- Gil-Gil, M.J.; Martinez-Garcia, M.; Sierra, A.; Conesa, G.; del Barco, S.; González-Jimenez, S.; Villà, S. Breast cancer brain metastases: A review of the literature and a current multidisciplinary management guideline. Clin. Transl. Oncol. 2014, 16, 436–446.
- Piezzo, M.; Chiodini, P.; Riemma, M.; Cocco, S.; Caputo, R.; Cianniello, D.; Di Gioia, G.; Di Lauro, V.; Rella, F.D.; Fusco, G.; et al. Progression-Free Survival and Overall Survival of CDK 4/6 Inhibitors Plus Endocrine Therapy in Metastatic Breast Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 6400.
- Brastianos, P.K.; Kim, A.E.; Wang, N.; Lee, E.Q.; Ligibel, J.; Cohen, J.V.; Chukwueke, U.N.; Mahar, M.; Oh, K.; White, M.D.; et al. Palbociclib demonstrates intracranial activity in progressive brain metastases harboring cyclin-dependent kinase pathway alterations. Nat. Cancer 2021, 2, 498–502.
- Bidard, F.C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J. Clin. Oncol. 2022, 40, 3246–3256.
- Robertson, J.F.R.; Bondarenko, I.M.; Trishkina, E.; Dvorkin, M.; Panasci, L.; Manikhas, A.; Shparyk, Y.; Cardona-Huerta, S.; Cheung, K.L.; Philco-Salas, M.J.; et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): An international, randomised, double-blind, phase 3 trial. Lancet 2016, 388, 2997–3005.
- Jones, R.H.; Casbard, A.; Carucci, M.; Cox, C.; Butler, R.; Alchami, F.; Madden, T.-A.; Bale, C.; Bezecny, P.; Joffe, J.; et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): A multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020, 21, 345–357.
- Howell, S.J.; Casbard, A.; Carucci, M.; Ingarfield, K.; Butler, R.; Morgan, S.; Meissner, M.; Bale, C.; Bezecny, P.; Moon, S.; et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive, HER2-negative breast cancer (FAKTION): Overall survival, updated progression-free survival, and expanded biomarker analysis from a randomised, phase 2 trial. Lancet Oncol. 2022, 23, 851–864.
- Turner, N.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortés, J.; Gomez, H.; Hu, X.; Jhaveri, K.; Loibl, S.; Murillo, S.M.; et al. Abstract GS3-04: GS3-04 Capivasertib and fulvestrant for patients with aromatase inhibitor-resistant hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: Results from the Phase III CAPItello-291 trial. Cancer Res. 2023, 83, GS3-04.
- Fitzgerald, D.M.; Muzikansky, A.; Pinto, C.; Henderson, L.; Walmsley, C.; Allen, R.; Ferraro, G.B.; Isakoff, S.; Moy, B.; Oh, K.; et al. Association between PIK3CA mutation status and development of brain metastases in HR+/HER2- metastatic breast cancer. Ann. Oncol. 2019, 30, v110.
- Huang, R.S.P.; Haberberger, J.; McGregor, K.; Mata, D.A.; Decker, B.; Hiemenz, M.C.; Lechpammer, M.; Danziger, N.; Schiavone, K.; Creeden, J.; et al. Clinicopathologic and Genomic Landscape of Breast Carcinoma Brain Metastases. Oncologist 2021, 26, 835–844.
- Miller, J.; Armgardt, E.; Svoboda, A. The efficacy and safety of alpelisib in breast cancer: A real-world analysis. J. Oncol. Pharm. Pract. 2022, 10781552221096413.
- Batalini, F.; Moulder, S.L.; Winer, E.P.; Rugo, H.S.; Lin, N.U.; Wulf, G.M. Response of Brain Metastases from PIK3CA-Mutant Breast Cancer to Alpelisib. JCO Precis. Oncol. 2020, 4, PO.19.00403.
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of Triple-Negative Breast Cancer. Annu. Rev. Pathol. 2022, 17, 181–204.
- Luo, Z.; Wu, S.; Zhou, J.; Xu, W.; Xu, Q.; Lu, L.; Xie, C.; Liu, Y.; Lu, W. All-stage targeted therapy for the brain metastasis from triple-negative breast cancer. Acta Pharm. Sin. B 2023, 13, 359–371.
- Corso, G.; D’Ecclesiis, O.; Magnoni, F.; Mazzotta, E.; Conforti, F.; Veronesi, P.; Sajjadi, E.; Venetis, K.; Fusco, N.; Gandini, S. Metaplastic breast cancers and triple-negative breast cancers of no special type: Are they prognostically different? A systematic review and meta-analysis. Eur. J. Cancer Prev. 2022, 31, 459–466.
- Corti, C.; Venetis, K.; Sajjadi, E.; Zattoni, L.; Curigliano, G.; Fusco, N. CAR-T cell therapy for triple-negative breast cancer and other solid tumors: Preclinical and clinical progress. Expert. Opin. Investig. Drugs 2022, 31, 593–605.
- Fusco, N.; Sajjadi, E.; Venetis, K.; Ivanova, M.; Andaloro, S.; Guerini-Rocco, E.; Montagna, E.; Caldarella, P.; Veronesi, P.; Colleoni, M.; et al. Low-risk triple-negative breast cancers: Clinico-pathological and molecular features. Crit. Rev. Oncol. Hematol. 2022, 172, 103643.
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769.
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158.
- di Mauro, P.; Schivardi, G.; Pedersini, R.; Laini, L.; Esposito, A.; Amoroso, V.; Laganà, M.; Grisanti, S.; Cosentini, D.; Berruti, A. Sacituzumab govitecan and radiotherapy in metastatic, triple-negative, and BRCA-mutant breast cancer patient with active brain metastases: A case report. Front. Oncol. 2023, 13, 1139372.
- Garber, H.R.; Raghavendra, A.S.; Lehner, M.; Qiao, W.; Gutierrez-Barrera, A.M.; Tripathy, D.; Arun, B.; Ibrahim, N.K. Incidence and impact of brain metastasis in patients with hereditary BRCA1 or BRCA2 mutated invasive breast cancer. NPJ Breast Cancer 2022, 8, 46.
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763.
- Litton, J.K.; Hurvitz, S.A.; Mina, L.A.; Rugo, H.S.; Lee, K.H.; Gonçalves, A.; Diab, S.; Woodward, N.; Goodwin, A.; Yerushalmi, R.; et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: Final overall survival results from the EMBRACA trial. Ann. Oncol. 2020, 31, 1526–1535.
- Senkus, E.; Delaloge, S.; Domchek, S.M.; Conte, P.; Im, S.A.; Xu, B.; Armstrong, A.; Masuda, N.; Fielding, A.; Robson, M.; et al. Olaparib efficacy in patients with germline BRCA-mutated, HER2-negative metastatic breast cancer: Subgroup analyses from the phase III OlympiAD trial. Int. J. Cancer 2023, 1–12.
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404.
- Agostinetto, E.; Losurdo, A.; Nader-Marta, G.; Santoro, A.; Punie, K.; Barroso, R.; Popovic, L.; Solinas, C.; Kok, M.; de Azambuja, E.; et al. Progress and pitfalls in the use of immunotherapy for patients with triple negative breast cancer. Expert. Opin. Investig. Drugs 2022, 31, 567–591.
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 994–1004.
- Rozenblit, M.; Huang, R.; Danziger, N.; Hegde, P.; Alexander, B.; Ramkissoon, S.; Blenman, K.; Ross, J.S.; Rimm, D.L.; Pusztai, L. Comparison of PD-L1 protein expression between primary tumors and metastatic lesions in triple negative breast cancers. J. Immunother. Cancer 2020, 8, e001558.
- Sajjadi, E.; Venetis, K.; Scatena, C.; Fusco, N. Biomarkers for precision immunotherapy in the metastatic setting: Hope or reality? Ecancermedicalscience 2020, 14, 1150.
- Cortes, J.; Cescon, D.W.; Rugo, H.S. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J. Clin. Oncol. 2020, 38, 1000.
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226.
- Kadamkulam Syriac, A.; Nandu, N.S.; Leone, J.P. Central Nervous System Metastases from Triple-Negative Breast Cancer: Current Treatments and Future Prospective. Breast Cancer (Dove Med. Press) 2022, 14, 1–13.
This entry is offline, you can click here to edit this entry!
