The advance in technology allows for the development of different CT scanners in the field of dual-energy computed tomography (DECT). In particular, a recently developed detector-based technology can collect data from different energy levels, thanks to its layers. The use of this system is suited for material decomposition with perfect spatial and temporal registration. Thanks to post-processing techniques, these scanners can generate conventional, material decomposition (including virtual non-contrast (VNC), iodine maps, Z-effective imaging, and uric acid pair images) and virtual monoenergetic images (VMIs).
1. Introduction
Conventional single-energy computed tomography (SECT) is a diagnostic imaging technique that uses a polyenergetic X-ray beam from a single source that rotates around the patient’s body and a panel of detectors that records the radiation attenuated by the different densities of tissues, expressed in terms of Hounsfield Unit (HU).
Due to its fast acquisition and diagnostic accuracy, SECT has become the gold standard for the detection and assessment of different pathological entities. One of the limits of conventional SECT is that the characterization of tissues with a similar density is not always straightforward as, for instance, in the case of calcified plaques and iodinated blood within arterial vessels in angiographic studies. Moreover, SECT protocols frequently consist of repeated scanning before, during and after contrast injection, resulting in high-dose exposures.
Dual-energy CT (DECT) is a more recent technology that helps to overcome these limitations by acquiring data at two different energy levels to derive different tissue attenuations. Data obtained can be combined to generate images for routine clinical interpretation or more accurate material characterization [
1].
The main contributors to attenuation coefficients during CT scanning are the photoelectric effect and the Compton scattering. Whereas the latter is minimally dependent on photon energy and is mainly related to a material’s electron density, the photoelectric effect is strongly X-ray-energy-dependent and increases with a higher element’s atomic number (Z). The photoelectric effect can be calculated by comparing attenuation levels derived from two energy levels. Because of its dependency on Z, it is crucial for distinguishing different materials with similar attenuation in any energy level. This characteristic is defined as material decomposition and represents the basis for spectral proprieties in DECT imaging [
2]. Elements with high Z, such as iodine (Z = 53) or calcium (Z = 20), are susceptible to the photoelectric effect and have strong spectral properties. These elements present similar CT attenuation values in SECT due to their relative density.
Conversely, when exposed to different energy levels via DECT scanning, they interact in different ways, regardless of their density. This capability of differentiating structures with similar densities but different elemental compositions underlie multiple clinical applications of DECT scanning [
3]. On the contrary, soft-tissue anatomic structures, including muscles or parenchyma, have a low photoelectric effect and consequently demonstrate less variability in their attenuation values at different energy levels.
The datasets of the two energy levels can be obtained using multiple acquisition techniques [
4]. Depending upon how the two different X-ray energies are generated, DECTs are divided into two major groups: tube-based and detector-based. Two of the three leading DECT platforms currently in the market are tube-based: dual-source DECT (ds-DECT) (Somatom Drive/Somatom Definition Flash, Siemens Medical Solutions, Forchheim, Germany) and rapid kV-switching DECT (rs-DECT) (Revolution CT, GE Healthcare, Milwaukee, WI, USA; Aquilion ONE GENESIS Edition, Canon Medical Systems, Otawara, Japan). In the detector-based category, the dual-layer detector DECT (dl-DECT) (IQon spectral CT, Philips Healthcare, Eindhoven, The Netherlands) is the only currently available platform.
The first DECT scanner approved for clinical use was introduced into the market in 2006 and was based on a dual-source technique. These scanners consist of two detectors and two X-ray sources, a low-kV and a high-kV tube, with 90° orientation differences that scan simultaneously to achieve two energy spectra. Conversely, rs-DECT uses a single X-ray tube that rapidly alternates between low and high kV during its rotation (fast switching) and a single detector that registers information from both energies. The most recent technology is the ds-DECT, which was commercially introduced in 2016. It is based on a single energetic radiation tube associated with a detector panel, constituting two layers (sandwich detector) that simultaneously detect two energy levels.
2. Dual-Layer Detector Dual-Energy CT Technology
As mentioned before, in the dl-DECT scanner system, spectral separation is achieved at the detector level. This system takes advantage of the polychromatic nature of the beam produced with a single-energy source, combined with highly specialized detectors that consist of two layers with maximal sensitivity for different energies. The top (inner) layer preferentially absorbs low-energy photons by design, approximately 50% of the total incident photon flux. In contrast, the bottom (outer) layer absorbs the remaining photons, which are primarily high-energy ones [
5,
6] (
Figure 1).
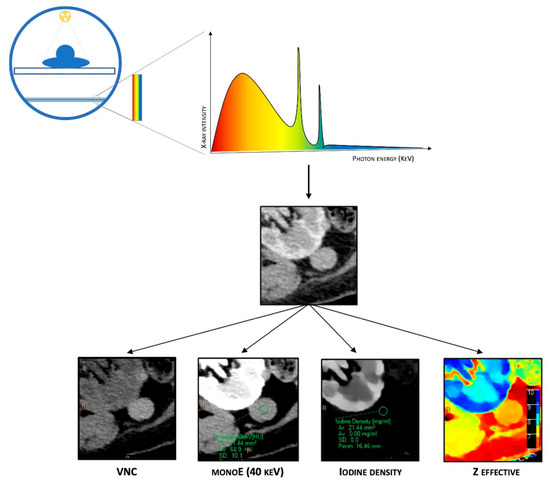
Figure 1. Schematic representation of DECT. It is based on a single energetic radiation tube associated with a detector panel constituted of two layers (sandwich detector) that simultaneously detect two energy levels. Different post-processing techniques are available due to spectral properties, such as material composition images (virtual non-contrast (VNC)), iodine maps, Z-effective imaging, and virtual monoenergetic images (VMIs).
A significant advantage of this system is, firstly, its excellent temporal registration. This system is well suited for material decomposition in the projection domain, making it quantitatively accurate and robust for possible patient motion. Another advantage is the perfect spatial registration of the acquired data to create a complete spectral dataset. The tube always operates at a high kVp, resulting in a high total X-ray power, which is advantageous for larger patients. Moreover, with this approach, scanning is performed at the full field of view of 50 cm. The last advantage is the dl-DECT retrospective acquisition mode: a dl-DECT scanner always acquires scans in the DECT mode, allowing one to gain spectral information for all scans performed, and hence there is no need to prospectively decide which scans perform in spectral mode, which is mandatory in other currently available dual-energy technologies. Retrospective on-demand spectral data of a region of interest allow radiologists to further investigate incidental findings without additional radiation exposure [
5,
7].
The main disadvantage of this system is its lower energy separation because the scintillator absorption properties do not offer a sharp distinction between lower- and higher-energy photons. As a result, the material differentiation contrast is decreased unless a higher radiation dose is used.
3. Dual-Layer CT Post-Processing
Combining data from both layers of detectors, dl-DECT scanners can generate conventional images comparable to those obtained from SECT, providing morphological details and material-specific image sets. Furthermore, plenty of different post-processing techniques are available due to spectral properties, such as material composition images (virtual non-contrast (VNC), iodine maps, Z-effective imaging, and uric acid pair images) and virtual monoenergetic images (VMIs). VNC images, also called “water-based”, are similar to conventional unenhanced CT images but are obtained via a dedicated algorithm that subtracts iodine-containing pixels from enhanced phases, allowing to create virtual plane images. Iodine concentration (IC) images (iodine maps) are material decomposition maps obtained via an algorithm that enhances only the pixels containing iodine. Iodine maps allow for identifying the presence or absence of iodine and its uptake in several tissues, which is particularly helpful in evaluating the contrast enhancement. Z-effective imaging consists of colorimetric maps that visually enhance the differences between tissues: the average atomic numbers of elements in each pixel are translated into color-coded images that provide a higher degree of discrimination than HU attenuation in conventional CT. Z-effective mapping is also used to define the peak enhancement (PE), which expresses the maximal concentration of the contrast agent with time in a tissue, according to the acquisition phase. Uric acid pair images show only pixels containing uric acid with original HU values, while all others appear dark, which is extremely useful for assessing urinary calculi composition and gout.
Finally, VMIs are a set of monochromatic images that simulate the appearance of images acquired using a monoenergetic X-ray beam at a selected energy level. VMIs can be obtained at discrete energy levels ranging from 40 to 190 keV with dl-DECT. Due to the approximation of the energy with the K-edge of iodine, low-keV VMIs show increased iodine conspicuity, which results in attenuation values equivalent to conventional images at a 120-kVp, but with a significant reduction in noise. Conversely, higher energy levels in VMIs reveal decreased iodine conspicuity and a drop in beam hardening artifacts, a physical phenomenon of the beam itself that produces an artifact that typically appears in the presence of metallic implants.
4. Radiation Dose
The White Paper of the Society of Computed Body Tomography on Dual-Energy CT published in 2016 stated that DECT acquisitions, even if using different X-ray spectra, do not provide additional radiation dose exposure in patients [
8].
In the literature, various studies have demonstrated similar or lower radiation dose exposure via DECT acquisitions compared to SECT [
9,
10,
11,
12]. One investigation revealed that DECT imaging at 80 and 140 kVp resulted in a decrease in the dose-length product and CT dose index values of 10% and 12%, respectively, compared to standard SECT (120 kVp) imaging using the same dual-source scanner, with no significant difference in objective image noise or subjective image quality [
9]. Duan et al. compared radiation dose and image quality for abdominal CT imaging performed on dl-DECT and conventional SECT scanners in patients of different sizes. The volume CT dose index (CTDIvol) during dl-DECT was similar to one measured on a conventional SECT for average-size patients, lower for smaller patients, and slightly higher for larger patients [
11].
Furthermore, VNC imaging allows one to reconstruct plain images from enhanced phases, reducing the number of scans and, consequently, the radiation dose [
13]. This tool is particularly advantageous in oncologic patients, who usually undergo repeated follow-up CT examinations, and pediatric patients.
Finally, a potential radiation dose reduction can be achieved by avoiding additional CT studies for further incidental lesion characterization.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13101740